Table 1.
Catalytic asymmetric synthesis of pyrroloindolines.
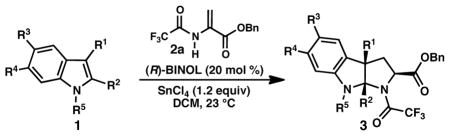 | ||
|---|---|---|
| entry | ee (%)a (exo/endo)b | |
| 1) |
 3a 86% yield dr = 4:1 |
94/91 |
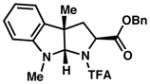
| 2) |
 3b 93% yield dr = 3:1 |
93/92 |
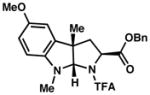
| 3) |
 3c 61% yield dr = 3:1 |
93/90 |
| 4) |
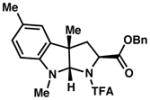 3d 84% yield dr = 5:1 |
94/91 |
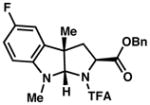
| 5) |
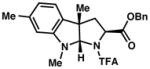 3e 91% yield dr = 4:1 |
94/90 |
| 6) |
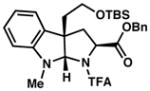 3f 54% yield dr = 6:1 |
92/90 |
| 7) |
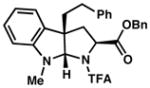 3g 80% yieldc dr = 4:1 |
92/90 |
| 8) |
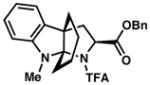 3h 65% yield dr = >18:1 |
86 |
| 9) |
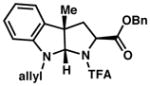 3i 90% yieldc dr = 3:1 |
93/90 |
| 10) |
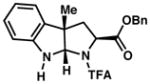 3j 18% yield dr = 8:1 |
95/93 |
Determined by chiral stationary phase SFC or HPLC.
Determined by 1H NMR analysis of mixture.
1.6 equiv SnCl4 were employed.
