Table 2.
Optimization of reaction parameters.a
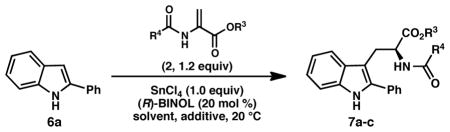 | ||||||
|---|---|---|---|---|---|---|
| entry | R3, R4 | pdt | solvent | additive | yield (%)b | ee (%)c |
| 1 | Bn, CF3 (2a) | 7a | DCM | -- | 12 | 35 |
| 2 | Me, CF3 (2b) | 7b | DCM | -- | 12 | 42 |
| 3 | Me, Me (2c) | 7c | DCM | -- | 73 | 78 |
| 4 | Me, Me (2c) | 7c | DCM | --d | 13 | -- |
| 5 | Me, Me (2c) | 7c | DCM | --e | 0 | -- |
| 6 | Me, Me (2c) | 7c | DCM | K2CO3 | 73 | 78 |
| 7 | Me, Me (2c) | 7c | DCM | 2,6-lutidine | 0 | -- |
| 8 | Me, Me (2c) | 7c | DCM | 4Å MS | 86 | 81 |
| 9 | Me, Me (2c) | 7c | DCE | 4Å MS | 87 | 79 |
| 10 | Me, Me (2c) | 7c | CHCl3 | 4Å MS | 80 | 72 |
Reactions conducted under inert atmosphere on 0.2 mmol scale for 2 h.
Isolated yield.
Determined by chiral stationary phase SFC.
No (R)-BINOL was employed.
No SnCl4was employed.
