Table 4.
Substrate scope of the tandem Friedel–Crafts conjugate addition/asymmetric protonation.a
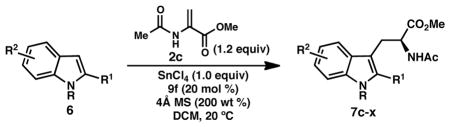 | |
|---|---|
| entry | |
|
|
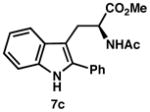
| 1) | 76% yield, 93% ee |
|
|
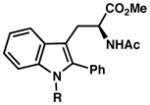
| 2) | R = Me (7d) 63% yield, 85% ee |
| 3) | R = allyl (7e) 68% yield, 85% ee |
|
|
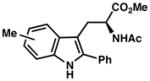
| 4) | 4-Me (7f): 88% yield, 96% ee |
| 5) | 5-Me (7g): 83% yield, 95% ee |
| 6) | 6-Me (7h): 80% yield, 89% ee |
| 7) | 7-Me (7i): 94% yield, 94% ee |
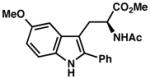 7j |
|
| 8) | 85% yield, 91% ee |
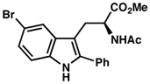 7k |
|
| 9)b | 60% yield, 93% ee |
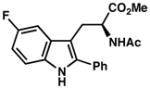 7l |
|
| 10)b | 63% yield, 92% ee |
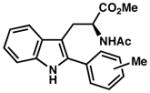
|
|
| 11) | 4-Me (7m): 86% yield, 94% ee |
| 12) | 2-Me (7n): 26% yield, 87% ee |
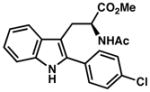 7o |
|
| 13) | 75% yield, 93% ee |
|
|
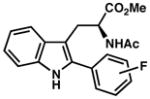
| 14) | 4-F (7p): 78% yield, 93% ee |
| 15) | 3-F (7q): 76% yield, 92% ee |
| 16) | 2-F (7r): 35% yield, 92% ee |
 7s |
|
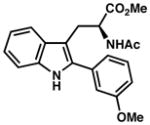
| 17) | 88% yield, 92% ee |
|
|
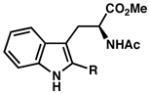
| 18) | R = Me (7t): 61% yield, 85% ee |
| 19) | R = n-Bu (7u): 72% yield, 91% ee |
| 20) | R = i-Pr (7v): 66% yield, 92% ee |
| 21) | R = t-Bu (7w): 29% yield, 84% ee |
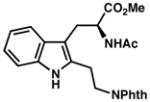 7x |
|
| 22) | 80% yield, 90% ee |
Reactions conducted under inert atmosphere on 0.1 or 0.2 mmol scale for 2 h. Isolated yields are reported. Enantiomeric excess was determined by chiral stationary phase SFC.
1.6 equiv SnCl4 were employed.
