Table 5.
Comparison of conditions for pyrroloindoline formation.
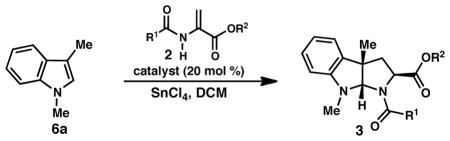 | ||||||
|---|---|---|---|---|---|---|
| entry | conditions | R1, R2 | pdt | yielda (%) | drb | ee (%)c |
| 1d | 9a | Me, Me (2c) | 3k | 70 | 5:1 | 65/80 |
| 2e | 9f, 4Å MS | Me, Me (2c) | 3k | 58 | 8:1 | 87/85 |
| 3d | 9a | CF3, Bn (2a) | 3a | 86 | 4:1 | 94/91 |
| 4e | 9f, 4Å MS | CF3, Bn (2a) | 3a | 39 | 7:1 | 98/95 |
Isolated yield.
Determined by 1H NMR analysis of mixture.
Determined by chiral stationary phase SFC or HPLC.
Reaction run with 1.0 equiv acrylate, 1.2 equiv SnCl4.
Reaction run with 1.2 equiv acrylate, 1.0 equiv SnCl4.
