Abstract
Background
Little is known about the use of warfarin in hemodialysis (HD) patients with atrial fibrillation (AF). We studied temporal trends of AF among older HD patients, and of warfarin use among those with AF.
Methods
We linked Medicare and prescription claims from older patients undergoing HD in two Eastern states. We established annual cohorts of prevalent HD patients; AF was ascertained from >2 claims (>7 days apart) in the same year with a diagnosis code indicating AF. Among those with AF, we defined current and past warfarin use. Demographic and clinical characteristics were also ascertained for each cohort. We used repeated-measures logistic regression to define the odds of AF and of current or past vs. absence of warfarin use.
Results
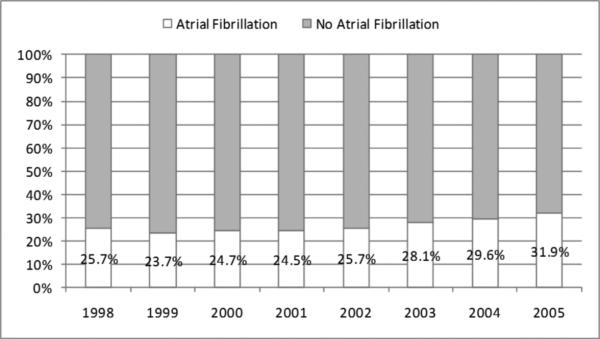
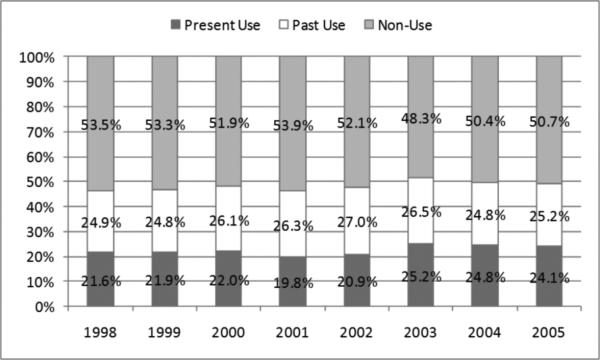
Of 6563 unique patients, 2185 were determined to have AF. The prevalence of AF increased from 26% in 1998 to 32% in 2005. In 2005, current warfarin use was present in 24% of AF patients and past use in 25%; 51% had no evidence of any warfarin use. No significant trends in utilization were observed from 1998-2005. Patients aged ≥85 years and non-whites were less likely to have received warfarin; most comorbidities were not associated with warfarin use except for patients with past pulmonary embolism or deep venous thrombosis who were more likely than those without such history.
Conclusion
While the prevalence of AF has been increasing among older HD patients, warfarin use was low and unchanged over time, perhaps reflecting the lack of evidence supporting such use.
Keywords: drug utilization, end-stage renal disease, anticoagulation
INTRODUCTION
Patients with chronic kidney disease requiring dialysis are particularly prone to developing atrial fibrillation (AF).(1-2) In the U.S., more than 10.5% of adults on hemodialysis had recurrent or persistent AF in 2006, a 3-fold increase since 1992.(2) Similar to the general population, age is a strong predictor for AF in patients undergoing hemodialysis, with patients over 65 years being, 4.5 to 7-fold more likely to have AF compared with patients under age 45.(2)
Patients undergoing hemodialysis are also at much increased risk for ischemic and hemorrhagic cerebrovascular events.(3) In the general population, prophylaxis of ischemic stroke using oral anticoagulation is recommended for patients with AF, especially among the elderly and patients with certain comorbidities.(4-5) Whether patients undergoing maintenance hemodialysis with AF should receive warfarin prophylaxis is controversial.(1, 6-10) In addition to the effects on ischemic and hemorrhagic stroke, this therapy may offer additional benefit in terms of vascular access patency. In contrast, anticoagulation in patients on hemodialysis increases risks of gastrointestinal and vascular access site bleeding,(11-12) and may accelerate the vascular calcification that often occurs in these patients.(6) The relationship of these risks and benefits and the direction of net benefit are currently unknown.
The existing data suggests relatively low rates of warfarin among patients not on hemodialysis.(13) Only 56% of Medicare beneficiaries over age 65 who had AF received warfarin treatment in 2002.(14) Little is known about the use of warfarin in older patients undergoing hemodialysis, especially about temporal trends in such use. We used a cohort of older hemodialysis patients who had prescription drug benefits through state insurance programs to study trends in AF and of warfarin use among those patients with AF.
METHODS
Source Population
Several datasets were merged to assemble the study population. Medicare claims of all individuals over the age of 65 in New Jersey and Pennsylvania between 1994 and 2005 were linked with drug claims data from three state-wide prescription benefit programs: New Jersey Medicaid, the New Jersey Pharmaceutical Assistance for the Aged and Disabled (PAAD), and the Pennsylvania Pharmaceutical Contract for the Elderly (PACE). The latter two programs provide generous prescription drug coverage to their residents that do not qualify for Medicaid, but who were still relatively indigent. Only patients enrolled both in Medicare and one of these prescription benefit programs were retained. These patients’ records were then augmented with claims from the United States Renal Data System which provides clinical information in the Medical Evidence Form, the treatment history file, and are a widely-accepted way of identifying patients with end-stage renal disease.
Study Cohorts
From the source population, we generated 8 consecutive annual cohorts (from 1998 to 2005) of prevalent patients undergoing hemodialysis. December 31 of each year was set as the index date. Patients were required to be stably undergoing maintenance hemodialysis as per USRDS TX_HIST60 variable and to be at least 66 years old on each index day to be included. We utilized all Medicare claims from 1 year prior to start of dialysis for each participant; these data were available even in newly incident patients due to the required age of ≥ 66 years on index. Participants were also required to have had active prescription drug coverage through one of the three programs as evidenced by the presence of at least one filled drug claim in the first and the second half of the respective calendar year.
Outcomes
Recurrent or Permanent Atrial Fibrillation
Atrial fibrillation was ascertained from diagnosis codes accompanying all medical claims. Presence of an International Classification of Diseases, 9th Revision (ICD-9) diagnosis code indicating atrial fibrillation as a primary or secondary diagnosis (ICD-9: 427.3#, where # can be any number or missing) has previously been shown to have a sensitivity of 97%, specificity of 99% and a positive predictive value (PPV) of 95%-96% for accurately identifying patients with atrial fibrillation compared with chart review.(15-17) This approach has been used successfully to identify Medicare patients for the National Registry of Atrial Fibrillation, and in several other claims-based research studies of AF.(18-20) Since we were interested in identifying patients with permanent or at least recurrent AF, we required presence of two such diagnosis codes at least 7 days apart to constitute AF. Patients were considered to have AF indefinitely from the date of the first diagnosis. Dialysis patients with AF are thought to have a rather small chance for successful cardioversion and long-term restoration of sinus rhythm, albeit quantitative data on this issue are lacking. We also conducted analyses that required 3 previous codes of AF to be subsequently considered an AF patient, or analyses that required “requalification” for AF each year from presence of 2 or more AF codes in the same year.
Warfarin Treatment
From all claims of filled prescriptions, we identified all that contained a National Drug Code identifier representing warfarin sodium (Coumadin). We identified the warfarin claim that most closely preceded each index date. Using the information on days supplied in the dispensing claim, we assessed whether a patient was a current user allowing a 15 day grace period after expiration of the medication days supplied. Since almost all prescriptions contained a 30 day supply, most patients who filled their most recent warfarin prescription on or after November 16 were considered current warfarin users. Other patients with a more distant warfarin dispensing were considered past users; patients without a warfarin prescription prior to the index date were labeled as non-users.
Other Variables
From USRDS, we ascertained demographic information: age on index, gender, race (Caucasian, African American, Asian American), and current Medicaid coverage. We calculated dialysis vintage (years since first ESRD treatment) and used information from the Medical Evidence Report form on whether a patient was unable to ambulate or transfer at initiation of dialysis and on reported underlying kidney disease. Comorbid conditions were ascertained from both the Medical Evidence Form and from Medicare claims. A comorbidity was considered present if noted on the Medical Evidence Form or if one inpatient or two outpatient encounters listed corresponding diagnosis codes. Presence of certain procedures codes also qualified patients for having a condition, e.g., amputation for peripheral artery disease. A comorbid condition was considered present indefinitely, once identified.
Statistical Analysis
We described relevant characteristics of each annual cohort using means and standard deviations for continuous, and counts and proportions for categorical data. We plotted the annual prevalence of atrial fibrillation and the proportion of current, past, and non-users of warfarin treatment among patients with atrial fibrillation. For the most recent cohort (December 31 2005), we used bivariable and multivariable logistic regression models to determine the crude and independent associations of each variable with the study outcomes. In addition, we analyzed data from all annual cohorts using repeated measures logistic regression models to assess the crude, demographics-adjusted, and fully adjusted temporal trends in the study outcomes using year as an ordinal variable. We also tested for interaction with time of all variables to understand any particular drivers of the hypothesized changes in the prevalence of the study outcomes.
This work was approved by the Institutional Review Boards of Brigham and Women's Hospital and Stanford University School of Medicine. Per requirement of the Centers for Medicare and Medicaid Services, cell counts less that 10 cannot be reported. Therefore, we removed all Native American patients from analysis (total N<10) and Asians and Blacks were collapsed into a single non-White race category for descriptive analyses.
RESULTS
Overall, 6563 unique patients contributed to this study. Of these 6563, 2185 were determined to have AF using our stated algorithm. The characteristics of each annual cohort, by presence of atrial fibrillation, are shown in Table 1. The prevalence of AF in HD patients over age 66 increased from 26% in 1998 to 32% in 2005 (Figure 1). The crude odds of AF increased by 5% (95% CI: 3%-7%) each year, which remained unchanged after adjustment for demographics, but attenuated to 3% per year after full adjustment (Table 2). More stringent definitions of AF yielded slightly lower annual prevalence estimates, but similar increases over time (data not shown).
Table 1.
Characteristics of Patients Undergoing Maintenance Hemodialysis on December 31, by Atrial Fibrillation
| 1998 | 1999 | 2000 | 2001 | |||||
|---|---|---|---|---|---|---|---|---|
| No AFib | AFib | No AFib | AFib | No AFib | AFib | No AFib | AFib | |
| Number (row %) | 1241 (74.3) | 430 (25.7) | 1324 (76.3) | 411 (23.7) | 1344 (75.3) | 441 (24.7) | 1381 (75.5) | 449 (24.5) |
| Age (years) | 75.4 (±6.1) | 76.6 (±6.4) | 75.4 (±6.1) | 77.3 (±6.3) | 75.5 (±6.2) | 77.0 (±6.5) | 75.8 (±6.2) | 77.23(±6.5) |
| Female (vs. Male) | 821 (66.2) | 275 (64.0) | 863 (65.2) | 259 (63.0) | 875 (65.1) | 280 (63.5) | 922 (66.8) | 300 (66.8) |
| White race (vs. non-white) | 729 (58.7) | 312 (72.6) | 783 (59.1) | 285 (69.3) | 789 (58.7) | 313 (71.0) | 802 (58.1) | 311 (49.3) |
| Medicaid (vs. non-Medicaid) | 455 (36.7) | 138 (32.1) | 516 (39.0) | 141 (34.3) | 517 (38.5) | 159 (36.1) | 512 (37.1) | 167 (37.2) |
| Dialysis vintage (years) | 2.9 (±2.8) | 3.0 (±2.7) | 3.0 (±2.8) | 3.5 (±3.2) | 3.1 (±3.0) | 3.5 (±3.2) | 3.2 (±3.1) | 3.6 (±3.1) |
| <1 year | 310 (25.0) | 83 (19.3) | 297 (22.4) | 71 (17.3) | 287 (21.4) | 80 (18.1) | 318 (23.0) | 91 (20.3) |
| 1-3 years | 492 (39.6) | 189 (44.0) | 542 (40.9) | 150 (36.5) | 537 (40.0) | 157 (35.6) | 511 (37.0) | 147 (32.7) |
| >3 years | 439 (35.4) | 158 (36.7) | 485 (36.6) | 190 (46.2) | 520 (38.7) | 204 (46.3) | 552 (40.0) | 211 (47.0) |
| Presumed cause of ESRD | ||||||||
| Diabetic nephropathy | 545 (43.9) | 181 (42.1) | 595 (44.9) | 165 (40.1) | 597 (44.4) | 168 (38.1) | 611 (44.2) | 180 (40.1) |
| Hypertensive nephrosclerosis | 428 (34.5) | 166 (38.6) | 455 (34.4) | 163 (39.7) | 458 (34.1) | 170 (38.5) | 471 (34.1) | 166 (37.0) |
| Glomerulonephritis | 105 (8.5) | 34 (7.9) | 118 (8.9) | 30 (7.3) | 115 (8.6) | 33 (7.5) | 118 (8.5) | 35 (7.8) |
| Other kidney disease | 163 (13.1) | 49 (11.4) | 156 (11.8) | 53 (12.9) | 174 (12.9) | 70 (15.9) | 181 (13.1) | 68 (15.1) |
| Presence of Comorbidity | ||||||||
| Diabetes | 834 (67.2) | 313 (72.8) | 905 (68.4) | 300 (73.0) | 907 (67.5) | 315 (71.4) | 960 (69.5) | 323 (71.9) |
| Hypertension | 1163 (93.7) | 417 (97.0) | 1245 (94.0) | 402 (97.8) | 1266 (94.2) | 433 (98.2) | 1324 (95.9) | 441 (98.2) |
| Congestive heart failure | 965 (77.8) | 398 (92.6) | 1034 (78.1) | 387 (94.2) | 1046 (77.8) | 416 (94.3) | 1043 (75.5) | 430 (95.8) |
| Coronary artery disease | 731 (58.9) | 325 (75.6) | 795 (60.0) | 329 (80.0) | 803 (59.7) | 347 (78.7) | 819 (59.3) | 344 (76.6) |
| Cerebrovascular disease | 426 (34.3) | 187 (43.5) | 443 (33.5) | 173 (42.1) | 452 (33.6) | 190 (43.1) | 474 (34.3) | 210 (46.8) |
| Peripheral artery disease | 692 (55.8) | 306 (71.2) | 751 (56.7) | 301 (73.2) | 756 (56.3) | 321 (72.8) | 763 (55.2) | 322 (71.7) |
| Chronic obstructive pulmonary disease | 353 (28.4) | 183 (42.6) | 388 (29.3) | 189 (46.0) | 400 (29.8) | 196 (44.4) | 410 (29.7) | 201 (44.8) |
| Cancer | 128 (10.3) | 53 (12.3) | 157 (11.9) | 53 (12.9) | 159 (11.8) | 67 (15.2) | 169 (12.2) | 63 (14.0) |
| 2002 | 2003 | 2004 | 2005 | |||||
|---|---|---|---|---|---|---|---|---|
| No AFib | AFib | No AFib | AFib | No AFib | AFib | No AFib | AFib | |
| Number (row %) | 1382 (74.3) | 478 (25.7) | 1372 (71.9) | 536 (28.1) | 1392 (70.4) | 585 (29.6) | 1391 (68.1) | 651 (31.9) |
| Age (years) | 76.0 (±6.4) | 77.4 (±6.6) | 75.8 (±6.5) | 77.3 (±6.8) | 76.2 (±6.6) | 77.3 (±6.6) | 76.0 (±6.7) | 77.7 (±6.6) |
| Female (vs. Male) | 878 (63.5) | 298 (62.3) | 867 (63.2) | 335 (62.5) | 883 (63.4) | 343 (58.6) | 860 (61.8) | 381 (58.5) |
| White race (vs. non-white) | 790 (57.2) | 344 (72.0) | 795 (57.9) | 388 (72.4) | 826 (59.3) | 439 (75.0) | 816 (58.7) | 477 (73.3) |
| Medicaid (vs. non-Medicaid) | 528 (38.2) | 142 (29.7) | 494 (36.0) | 168 (38.5) | 459 (33.0) | 173 (29.6) | 394 (28.3) | 187 (28.7) |
| Dialysis vintage (years) | 3.3 (±3.2) | 3.5 (±3.1) | 3.5 (±3.4) | 3.6 (±3.4) | 3.5 (±3.3) | 3.6 (±3.3) | 3.6 (±3.4) | 3.6 (±3.2) |
| <1 year | 288 (20.8) | 86 (18.0) | 281 (20.5) | 112 (20.9) | 285 (20.5) | 108 (18.5) | 292 (21.0) | 126 (19.4) |
| 1-3 years | 520 (37.6) | 173 (36.2) | 522 (38.0) | 199 (37.1) | 488 (35.1) | 209 (35.7) | 477 (34.3) | 243 (37.3) |
| >3 years | 574 (41.5) | 219 (45.8) | 569 (41.5) | 225 (42.0) | 619 (44.5) | 268 (45.8) | 622 (44.7) | 282 (43.3) |
| Presumed cause of ESRD | ||||||||
| Diabetic nephropathy | 611 (44.2) | 195 (40.8) | 618 (45.0) | 201 (37.5) | 620 (44.5) | 241 (41.2) | 652 (46.9) | 258 (39.6) |
| Hypertensive nephrosclerosis | 478 (34.6) | 174 (36.4) | 482 (35.1) | 208 (38.8) | 498 (35.8) | 225 (38.5) | 462 (33.2) | 257 (39.5) |
| Glomerulonephritis | 114 (8.2) | 42 (8.8) | 106 (7.7) | 51 (9.5) | 93 (6.7) | 49 (8.4) | 91 (6.5) | 55 (8.4) |
| Other kidney disease | 179 (13.0) | 67 (14.0) | 166 (12.1) | 76 (14.2) | 181 (13.0) | 70 (12.0) | 186 (13.4) | 81 (12.4) |
| Presence of Comorbidity | ||||||||
| Diabetes | 1021 (73.9) | 351 (73.4) | 1044 (76.1) | 410 (76.5) | 1063 (76.4) | 449 (76.8) | 1120 (80.5) | 531 (81.6) |
| Hypertension | 1341 (97.0) | 473 (99.0) | 1341 (97.7) | 533 (99.4) | 1373 (98.6) | 583 (99.7) | 1378 (99.1) | 649 (99.7) |
| Heart failure | 1083 (78.4) | 458 (95.8) | 1089 (79.4) | 507 (94.6) | 1097 (78.8) | 550 (94.0) | 1127 (81.0) | 620 (95.2) |
| Coronary artery disease | 851 (61.6) | 378 (79.1) | 854 (62.2) | 429 (80.0) | 841 (60.4) | 451 (77.1) | 868 (62.4) | 505 (77.6) |
| Cerebrovascular disease | 487 (35.2) | 220 (46.0) | 497 (36.2) | 210 (39.2) | 510 (36.6) | 268 (45.8) | 526 (37.8) | 314 (48.2) |
| Peripheral artery disease | 819 (59.3) | 337 (70.5) | 832 (60.6) | 375 (70.0) | 843 (60.6) | 428 (73.2) | 862 (62.0) | 478 (73.4) |
| Chronic obstructive pulmonary disease | 444 (32.1) | 234 (49.0) | 477 (34.8) | 248 (46.3) | 487 (35.0) | 292 (49.9) | 509 (36.6) | 335 (51.5) |
| Cancer | 181 (13.1) | 83 (17.4) | 190 (13.8) | 95 (17.7) | 198 (14.2) | 110 (18.8) | 237 (17.0) | 122 (18.7) |
Note: Per federal research regulations, data on patients who were unable to transfer and patients who were unable to ambulate are not reported, since some cell counts were ≤10. For the same reason, Asians and Blacks were collapsed into a “non-white” category. Data are presented as count (column %) or mean (± standard deviation), unless indicated otherwise.
Abbreviations: AFib – atrial fibrillation; ESRD – end-stage renal disease
Figure 1.
Trends in the Prevalence of Atrial Fibrillation among Older Patients Receiving Hemodialysis
Table 2.
Predictors of Prevalent Atrial Fibrillation
| 2005 Crude | 2005 Fully-adjusted | All Years Fully-adjusted | ||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | Odds Ratio | 95% CI | Odds Ratio | 95% CI | |
| Age (per year) | 1.04 | 1.02 – 1.05 | 1.02 | 1.01 – 1.04 | 1.02 | 1.01 – 1.03 |
| Female (vs. Male) | 0.85 | 0.69 – 1.03 | 0.83 | 0.67 – 1.02 | 0.92 | 0.81 – 1.05 |
| Race (vs. Caucasian) | ||||||
| African American | 0.53 | 0.42 – 0.66 | 0.52 | 0.41 – 0.67 | 0.60 | 0.55 – 0.65 |
| Asian American | 0.37 | 0.15 – 0.89 | 0.44 | 0.18 – 1.09 | 0.58 | 0.50 – 0.67 |
| Medicaid (vs. no Medicaid beneficiary) | 0.93 | 0.75 – 1.15 | 0.95 | 0.75 – 1.21 | 0.91 | 0.80 – 1.04 |
| Years since first ESRD treatment (per year) | 1.03 | 1.01 – 1.06 | 1.04 | 1.01 – 1.07 | 1.03 | 1.01 – 1.05 |
| Underlying kidney disease (vs. DN) | ||||||
| Hypertensive nephropathy | 1.33 | 1.07 – 1.65 | 1.32 | 1.03 – 1.71 | 1.28 | 1.09 – 1.50 |
| Glomerulonephritis | 1.58 | 1.09 – 2.29 | 1.50 | 0.99 – 2.28 | 1.29 | 1.00 – 1.66 |
| Other kidney disease | 1.12 | 0.82 – 1.52 | 1.09 | 0.76 – 1.55 | 1.28 | 1.03 – 1.59 |
| Comorbidities (vs. absence of comorbidity) | ||||||
| Diabetes | 1.02 | 0.80 – 1.31 | 1.11 | 0.83 – 1.48 | 1.13 | 0.97 – 1.32 |
| Hypertension | 5.42 | 0.71 – 41.3 | 2.87 | 0.36 – 22.8 | 1.48 | 0.97 – 2.25 |
| Heart failure | 4.64 | 3.06 – 7.03 | 3.40 | 2.20 – 5.25 | 3.40 | 2.70 – 4.27 |
| Coronary artery disease | 2.11 | 1.68 – 2.64 | 1.54 | 1.21 – 1.97 | 1.61 | 1.41 – 1.85 |
| Cerebrovasular disease | 1.58 | 1.30 – 1.92 | 1.38 | 1.12 – 1.70 | 1.20 | 1.06 – 1.35 |
| Peripheral artery disease | 1.66 | 1.34 – 2.05 | 1.15 | 0.91 – 1.46 | 1.30 | 1.15 – 1.48 |
| Chronic obstructive pulmonary disease | 1.85 | 1.52 – 2.25 | 1.45 | 1.18 – 1.79 | 1.42 | 1.26 – 1.60 |
| Cancer (excl. non-melanoma skin cancer) | 1.16 | 0.91 – 1.49 | 1.11 | 0.84 – 1.45 | 1.21 | 1.02 – 1.44 |
| Unable to ambulate | 1.46 | 0.85 – 2.50 | 1.20 | 0.64 – 2.25 | 1.58 | 1.10 – 2.27 |
| Unable to transfer | 1.30 | 0.44 – 3.83 | 1.13 | 0.32 – 3.92 | 0.98 | 0.52 – 1.87 |
| Calendar year | N.A. | N.A. | 1.03 | 1.00 – 1.05 | ||
Note: CI – confidence interval; DN – diabetic nephropathy; ESRD – end-stage renal disease
Table 2 shows the crude and fully adjusted associations of all variables with prevalent AF. Analyses utilizing data from all years yielded similar results to those solely relying on 2005 data, but the larger sample size led to increased precision. We found that older age was associated with higher prevalence of AF, with odds increasing by 2% per year (95% CI: 1%-3%). Compared with patients of white race, Blacks and Asians were each approximately 40% less likely to have AF. Longer duration since start of renal replacement therapy was associated with more AF, with a 3% increase in the odds of AF per additional year of dialysis. Most comorbid conditions were independently associated with AF, with heart failure having the strongest association (adjusted OR: 3.40; 95% CI: 2.70-4.27). These asociations did not differ materially when using any of the other definitions for the ascertainment of AF.(results not shown)
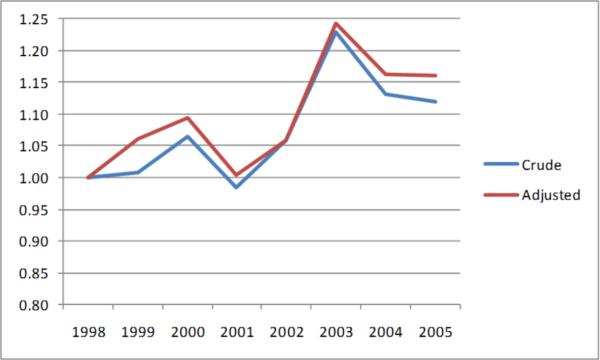
Among those 2185 individual patients with AF, of the proportion of patients that were considered current users of warfarin in each year, as ascertained from a recent (within 45 days) filled prescription for the drug, ranged from 20% to 25% (Figure 2). Additional 25% to 27% were past users, whereas approximately half of patients in each year were not observed to have used warfarin in the past. We observed no clear temporal trends in warfarin use and bivariable or fully-adjusted categorical analyses showed no significant differences to 1998 (e.g., the adjusted relative odds for warfarin use in 2005 compared with 1998 were 1.16; 95%CI: 0.89-1.51; Figure 3).
Figure 2.
Trends in Warfarin Use among Older Patients Receiving Hemodialysis who had Atrial Fibrillation
Figure 3.
Relative Odds of Warfarin Use over Time among Older Patients Receiving Hemodialysis who had Atrial Fibrillation
Note: Outcome is current or past versus never use of warfarin, odds ratios compare each year with 1998 as referent.
Characteristics of current or past versus non-users are displayed in Table 3. Older age was inversely associated with warfarin use, with patients aged 85 years or more having 29% (95% CI: 5%-47%) lower odds of warfarin use compared with otherwise similar seniors between 66 and <75 years (Table 4). Patients of non-white race had 41% (95% CI: 24%-54%) lower odds to receive warfarin compared with whites. Almost none of the comorbidities were associated with warfarin use, with the notable exceptions of past deep venous thrombosis (adjusted OR: 2.26; 95% CI: 1.81-2.84) and pulmonary embolism (adjusted OR: 1.31; 95% CI: 1.03-1.67).
Table 3.
Characteristics of Patients Undergoing Maintenance Hemodialysis with Atrial Fibrillation, on December 31, by Warfarin Use (Never vs. Current or Past)
| 1998 | 1999 | 2000 | 2001 | |||||
|---|---|---|---|---|---|---|---|---|
| No Warfarin | Any Warfarin | No Warfarin | Any Warfarin | No Warfarin | Any Warfarin | No Warfarin | Any Warfarin | |
| Number (row %) | 230 (53.5) | 200 (46.5) | 219 (53.3) | 192 (46.7) | 229 (51.9) | 212 (48.1) | 242 (53.9) | 207 (46.1) |
| Age (years) | 77.2 (±6.8) | 76.0 (±5.8) | 77.3 (±6.5) | 77.2 (±5.9) | 76.5 (±6.7) | 77.5 (±6.3) | 77.5 (±6.7) | 76.9 (±6.3) |
| Female (vs. Male) | 143 (62.2) | 132 (66.0) | 138 (63.0) | 121 (63.0) | 136 (59.4) | 144 (67.9) | 156 (64.5) | 144 (69.6) |
| White race (vs. non-white) | 149 (64.8) | 163 (81.5) | 134 (61.2) | 151 (78.6) | 146 (63.8) | 167 (78.8) | 155 (64.1) | 156 (75.4) |
| Medicaid (vs. non-Medicaid) | 84 (36.5) | 54 (27.0) | 86 (39.3) | 55 (28.6) | 90 (39.3) | 69 (32.5) | 102 (42.2) | 65 (31.4) |
| Dialysis vintage (years) | 3.2 (±2.9) | 2.7 (±2.4) | 4.0 (±3.8) | 3.0 (±2.2) | 4.0 (±3.7) | 3.0 (±2.4) | 3.6 (±3.0) | 3.6 (±3.2) |
| <1 year | 36 (15.7) | 47 (23.5) | 33 (15.1) | 38 (19.8) | 35 (15.3) | 45 (21.2) | 52 (21.5) | 39 (18.8) |
| 1-3 years | 99 (43.0) | 90 (45.0) | 74 (33.8) | 76 (39.6) | 77 (33.6) | 80 (37.7) | 76 (31.4) | 71 (34.3) |
| >3 years | 95 (41.3) | 63 (31.5) | 112 (51.1) | 78 (40.6) | 117 (51.1) | 87 (41.0) | 114 (47.1) | 97 (46.9) |
| Presence of Comorbidity | ||||||||
| Diabetes | 164 (71.3) | 149 (74.5) | 157 (71.7) | 143 (74.5) | 165 (72.1) | 150 (70.8) | 182 (75.2) | 141 (68.1) |
| Hypertension | 223 (97.0) | 194 (97.0) | 214 (97.7) | 188 (97.9) | 225 (98.3) | 208 (98.1) | 289 (98.8) | 202 (97.6) |
| Heart failure | 215 (93.5) | 183 (91.5) | 204 (93.2) | 183 (95.3) | 213 (93.0) | 203 (95.8) | 230 (95.0) | 200 (96.6) |
| Coronary artery disease | 173 (75.2) | 152 (76.0) | 176 (80.4) | 153 (79.7) | 176 (76.9) | 171 (80.7) | 182 (75.2) | 162 (78.3) |
| Cerebrovascular disease | 98 (42.6) | 106 (53.0) | 101 (46.1) | 99 (51.3) | 113 (49.3) | 105 (49.5) | 125 (51.7) | 110 (53.1) |
| Peripheral artery disease | 165 (71.7) | 141 (70.5) | 158 (72.2) | 143 (74.5) | 163 (71.2) | 158 (74.5) | 172 (71.1) | 150 (72.5) |
| Chronic obstructive pulmonary disease | 96 (41.7) | 87 (43.5) | 105 (48.0) | 84 (43.8) | 105 (45.9) | 91 (42.9) | 105 (43.4) | 96 (46.4) |
| Cancer | 30 (13.0) | 23 (11.5) | 26 (11.9) | 27 (14.1) | 36 (15.7) | 31 (14.6) | 38 (15.7) | 25 (12.1) |
| Gastrointestinal bleed | 124 (53.9) | 84 (42.0) | 121 (55.3) | 95 (49.5) | 116 (50.7) | 98 (46.2) | 109 (45.0) | 101 (48.8) |
| Peptic ulcer disease | 49 (21.3) | 32 (16.0) | 50 (22.8) | 40 (20.8) | 41 (17.9) | 30 (14.2) | 40 (16.5) | 34 (16.4) |
| Liver disease | 22 (9.6) | 12 (6.0) | 23 (10.5) | 18 (9.4) | 34 (14.9) | 12 (5.7) | 23 (9.5) | 15 (7.2) |
| Dementia | 40 (17.4) | 28 (14.0) | 36 (16.4) | 31 (16.1) | 36 (15.7) | 23 (10.8) | 39 (16.1) | 19 (9.2) |
| Depression | 46 (20.0) | 45 (22.5) | 42 (19.2) | 36 (18.8) | 55 (24.0) | 31 (14.6) | 63 (26.0) | 42 (20.3) |
| Prior nursing home stay | 108 (47.0) | 102 (51.0) | 109 (49.8) | 86 (44.8) | 115 (50.2) | 100 (47.2) | 125 (51.7) | 91 (44.0) |
| Prior fall or fracture | 46 (20.0) | 34 (17.0) | 42 (19.2) | 33 (17.2) | 48 (22.0) | 31 (14.6) | 44 (18.2) | 45 (21.7) |
| Deep venous thrombosis | 48 (20.9) | 66 (38.0) | 50 (22.8) | 65 (33.9) | 54 (23.6) | 70 (33.0) | 54 (22.3) | 76 (36.7) |
| Pulmonary embolism | 73 (31.7) | 61 (30.5) | 56 (25.6) | 61 (31.8) | 50 (21.8) | 64 (30.2) | 49 (20.3) | 60 (29.0) |
| Valvular disease or repair | 115 (50.0) | 111 (55.5) | 117 (53.4) | 108 (56.3) | 118 (51.5) | 119 (56.1) | 140 (57.9) | 120 (58.0) |
| History of vascular thrombosis | 181 (78.7) | 161 (80.5) | 182 (83.1) | 157 (81.1) | 181 (79.0) | 158 (74.5) | 193 (79.8) | 158 (76.3) |
| 2002 | 2003 | 2004 | 2005 | |||||
|---|---|---|---|---|---|---|---|---|
| No Warfarin | Any Warfarin | No Warfarin | Any Warfarin | No Warfarin | Any Warfarin | No Warfarin | Any Warfarin | |
| Number (row %) | 249 (52.1) | 229 (47.9) | 259 (48.3) | 277 (51.7) | 295 (50.4) | 290 (49.6) | 330 (50.7) | 321 (49.3) |
| Age (years) | 77.7 (±6.6) | 77.2 (±6.5) | 77.7 (±6.9) | 77.0 (±6.6) | 77.8 (±6.6) | 76.8 (±6.6) | 78.0 (±6.6) | 77.5 (±6.6) |
| Female (vs. Male) | 156 (62.7) | 142 (62.0) | 160 (61.8) | 175 (63.2) | 174 (59.0) | 169 (58.3) | 198 (60.0) | 183 (57.0) |
| White race (vs. non-white) | 166 (66.7) | 178 (77.7) | 178 (68.7) | 210 (75.8) | 211 (71.5) | 228 (78.6) | 230 (69.7) | 247 (76.9) |
| Medicaid (vs. non-Medicaid) | 80 (32.1) | 62 (27.1) | 81 (31.3) | 87 (31.4) | 90 (30.5) | 83 (28.6) | 99 (30.0) | 88 (27.4) |
| Dialysis vintage (years) | 3.5 (±3.1) | 3.5 (±3.2) | 3.5 (±3.2) | 3.6 (±3.5) | 3.8 (±3.3) | 3.5 (±3.2) | 3.6 (±3.1) | 3.6 (±3.2) |
| bku<1 year | 47 (17.7) | 42 (18.3) | 53 (20.5) | 59 (21.3) | 59 (20.0) | 49 (16.9) | 62 (18.8) | 64 (19.9) |
| bku1-3 years | 91 (36.6) | 82 (35.8) | 97 (37.5) | 104 (37.5) | 95 (32.2) | 114 (39.3) | 123 (37.3) | 120 (37.4) |
| bku>3 years | 114 (45.8) | 105 (45.9) | 109 (42.1) | 116 (41.9) | 141 (47.8) | 127 (43.8) | 145 (43.9) | 137 (42.7) |
| Presence of Comorbidity | ||||||||
| Diabetes | 192 (77.1) | 159 (69.4) | 203 (78.4) | 207 (74.7) | 229 (77.6) | 220 (75.9) | 271 (82.1) | 260 (81.0) |
| Hypertension | 246 (98.8) | 227 (99.1) | 258 (99.6) | 275 (99.3) | 294 (99.7) | 289 (99.7) | 329 (99.7) | 320 (99.7) |
| Heart failure | 237 (95.2) | 221 (96.5) | 245 (94.6) | 262 (94.6) | 276 (93.6) | 274 (94.5) | 313 (94.9) | 307 (95.6) |
| Coronary artery disease | 191 (76.7) | 187 (81.7) | 209 (80.7) | 220 (79.4) | 227 (77.0) | 224 (77.2) | 254 (77.0) | 251 (78.2) |
| Cerebrovascular disease | 130 (52.2) | 116 (50.7) | 119 (46.0) | 125 (45.1) | 151 (51.2) | 151 (52.1) | 193 (58.5) | 173 (53.9) |
| Peripheral artery disease | 173 (69.5) | 164 (71.6) | 177 (68.3) | 198 (71.5) | 209 (70.9) | 219 (75.5) | 235 (71.2) | 243 (75.7) |
| Chronic obstructive pulmonary disease | 119 (47.8) | 115 (50.2) | 116 (44.8) | 132 (47.7) | 138 (46.8) | 154 (53.1) | 154 (46.7) | 181 (56.4) |
| Cancer | 37 (14.9) | 46 (20.1) | 45 (17.4) | 50 (18.1) | 57 (19.3) | 53 (18.3) | 59 (17.9) | 63 (19.6) |
| Gastrointestinal bleed | 107 (43.0) | 95 (41.5) | 124 (47.9) | 123 (44.4) | 158 (53.6) | 126 (43.4) | 165 (50.0) | 160 (49.8) |
| Peptic ulcer disease | 36 (14.5) | 28 (12.2) | 32 (12.4) | 40 (14.4) | 45 (15.3) | 24 (8.3) | 46 (13.9) | 37 (11.5) |
| Liver disease | 16 (6.4) | 11 (4.8) | 15 (5.8) | 10 (3.6) | 21 (7.1) | 15 (5.2) | 19 (5.8) | 12 (3.7) |
| Dementia | 41 (16.5) | 22 (9.6) | 30 (11.6) | 20 (7.2) | 53 (18.0) | 41 (14.1) | 68 (20.6) | 58 (18.1) |
| Depression | 50 (20.1) | 41 (17.9) | 58 (22.4) | 68 (24.5) | 71 (24.1) | 88 (30.3) | 84 (25.5) | 92 (28.7) |
| Prior nursing home stay | 131 (52.6) | 108 (47.2) | 141 (54.4) | 152 (54.9) | 180 (61.0) | 165 (56.9) | 209 (63.3) | 192 (59.8) |
| Prior fall or fracture | 52 (20.9) | 45 (19.7) | 60 (23.2) | 57 (20.6) | 62 (21.0) | 57 (19.7) | 72 (21.8) | 63 (19.6) |
| Deep venous thrombosis | 43 (17.3) | 88 (38.4) | 46 (17.8) | 107 (38.6) | 62 (21.0) | 99 (34.1) | 72 (21.8) | 117 (36.4) |
| Pulmonary embolism | 50 (20.1) | 71 (31.0) | 49 (18.9) | 89 (32.1) | 67 (22.7) | 71 (24.5) | 59 (17.9) | 78 (24.3) |
| Valvular disease or repair | 150 (60.2) | 130 (56.8) | 158 (61.0) | 163 (58.8) | 170 (57.6) | 198 (68.3) | 215 (65.2) | 233 (72.6) |
| History of vascular thrombosis | 200 (80.3) | 189 (82.5) | 214 (82.6) | 230 (83.0) | 250 (84.8) | 245 (84.5) | 283 (85.8) | 269 (83.8) |
Note: Per federal research regulations, data on patients who were unable to transfer, patients who were unable to ambulate, and on prior intracranial bleed are not reported, since some cell counts were ≤10. For the same reason, Asians and Blacks were collapsed into a “non-white” category. Data are presented as count (column %) or mean (± standard deviation), unless indicated otherwise.
Abbreviations: AFib – atrial fibrillation; ESRD – end-stage renal disease
Table 4.
Predictors of Current or Past Warfarin Use in Hemodialysis Patients with Atrial Fibrillation
| Crude | Fully-adjusted | |||
|---|---|---|---|---|
| Odds Ratio | 95% CI | Odds Ratio | 95% CI | |
| Age 75 to <85 years (vs. 66 to <75 years) | 0.99 | 0.81 – 1.21 | 0.93 | 0.75 – 1.15 |
| Age ≥ 85 years (vs. 66 to <75 years) | 0.79 | 0.60 – 1.05 | 0.71 | 0.53 – 0.95 |
| Female (vs. Male) | 1.06 | 0.87 – 1.29 | 1.04 | 0.85 – 1.29 |
| Non-White Race (vs. Caucasian) | 0.57 | 0.45 – 0.72 | 0.59 | 0.46 – 0.76 |
| Medicaid (vs. no Medicaid beneficiary) | 0.78 | 0.63 – 0.95 | 0.86 | 0.69 – 1.07 |
| 1-3 years (vs. <1 year since since first ESRD treatment) | 0.98 | 0.84 – 1.15 | 0.96 | 0.81 – 1.14 |
| >3 years (vs. <1 year since since first ESRD treatment) | 0.84 | 0.68 – 1.02 | 0.83 | 0.66 – 1.05 |
| Comorbidities (vs. absence of comorbidity) | ||||
| Diabetes | 0.90 | 0.72 – 1.12 | 0.91 | 0.72 – 1.14 |
| Hypertension | 0.94 | 0.42 – 2.08 | 0.96 | 0.45 – 2.05 |
| Heart failure | 1.20 | 0.80 – 1.79 | 1.18 | 0.77 – 1.81 |
| Coronary artery disease | 1.09 | 0.87 – 1.36 | 1.02 | 0.81 – 1.28 |
| Peripheral artery disease | 1.14 | 0.93 – 1.40 | 1.12 | 0.91 – 1.39 |
| Chronic obstructive pulmonary disease | 1.13 | 0.94 – 1.36 | 1.09 | 0.90 – 1.33 |
| Cancer (excl. non-melanoma skin cancer) | 1.04 | 0.80 – 1.34 | 1.06 | 0.81 – 1.39 |
| Cerebral Ischemia | 1.04 | 0.87 – 1.24 | 1.07 | 0.88 – 1.30 |
| Intracranial bleed | 1.05 | 0.66 – 1.67 | 1.05 | 0.64 – 1.73 |
| Gastrointestinal bleed | 0.84 | 0.70 – 1.01 | 0.87 | 0.71 – 1.06 |
| Peptic ulcer disease | 0.81 | 0.62 – 1.05 | 0.88 | 0.66 – 1.18 |
| Liver disease | 0.63 | 0.42 – 0.93 | 0.67 | 0.45 – 1.00 |
| Dementia | 0.72 | 0.56 – 0.91 | 0.81 | 0.62 – 1.06 |
| Depression | 1.01 | 0.81 – 1.25 | 0.98 | 0.78 – 1.23 |
| History of falls or fractures | 0.90 | 0.72 – 1.12 | 0.84 | 0.66 – 1.06 |
| Prior nursing home stay | 0.88 | 0.73 – 1.05 | 0.82 | 0.67 – 1.01 |
| Deep venous thrombosis | 2.10 | 1.70 – 2.59 | 2.26 | 1.81 – 2.84 |
| Pulmonary embolism | 1.43 | 1.14 – 1.78 | 1.31 | 1.03 – 1.67 |
| Valvular disease or repair | 1.17 | 0.97 – 1.40 | 1.10 | 0.91 – 1.35 |
| History of vascular thrombosis | 0.95 | 0.76 – 1.18 | 0.86 | 0.67 – 1.09 |
Multivariable model also adjusted for calendar year. Crude and adjusted associations of calendar year with current or past warfarin use are shown in Figure 3. Abbreviation: ESRD – end-stage renal disease.
DISCUSSION
We observed an increasing prevalence of AF among older hemodialysis patients, reaching 32% in 2005. Among those with AF, less than half had evidence of warfarin use, and only approximately 25% were current users. We did not detect any significant trends towards increased use of warfarin over the 8 years of our study.
The evidence supporting oral anticoagulation in dialysis patients with AF is weak and there is concern that evidence from the general population cannot be extrapolated to patients undergoing chronic dialysis. In 2005, the Kidney/Dialysis Outcomes and Quality Initiative (KDOQI) cardiovascular guidelines indicated that the recommendations of the guidelines by the American Heart Association for the overall population should be followed in the care of dialysis patients.(4, 21) In was noted, however, that use of warfarin should be accompanied with heightened monitoring in these patients because of their increased risks for thrombotic and hemorrhagic events.
While most of the studies that formally evaluated the outcomes of warfarin in dialysis patients with AF have been small and methodologically limited, two larger studies have recently tested the association between warfarin use and the risk of stroke. One study enrolled incident hemodialysis patients of a national provider into a retrospective cohort study and distinguished among patients who had AF at the time they initiated dialysis from those who did not have AF.(9) Among the 1671 incident hemodialysis patients with pre-existing AF, warfarin users had more than twice the risk of ischemic stroke compared with non-users. Another analysis using the Dialysis Outcomes and Practice Pattern Study employed a similar approach and found the risk of stroke 2.2-fold elevated among 1107 dialysis patients with AF over age 75 years who were warfarin users compared with non-users; no significant association was found, however, among 1137 patients between 65-74 years and among 1001 patients aged 65 years or younger (both hazard ratios were ~1.3).(1) Neither of the studies observed the incidence of AF, which is when the treatment decision for AF is being made, and both studies relied heavily on existing users of warfarin. Such an approach is highly prone to bias,(22) since relevant variables at the time of diagnosis and treatment initiation are unobserved and any unrelated indications or contraindication for anticoagulation (or discontinuations of treatment) cannot be considered.
It is unusual that virtually no disease-characteristics correlated with warfarin use in our study, with the exception of past deep venous thrombosis and pulmonary embolism. These conditions are both separate and strong indications for warfarin but are unrelated to the risk of AF-related stroke. It is possible that the lack of specific evidence in dialysis patients creates a situation of therapeutic equipoise, where the predominant driver of treatment is provider preference or belief rather than patient factors. Unfortunately, our study is too small to study the importance of patient vs. provider factors in predicting anticoagulant use.
There are several limitations to our analysis. While the validity of our study is strengthened by the reliable ascertainment of warfarin use from complete prescription drug claims, but we are unable to distinguish the specific reason or indication for warfarin use such as the prevention of vascular access thrombosis which is a a non-evidence-based practice that exposes at-risk patients to often subtherapeutic doses of warfarin. Thus, the proportion of therapeutic anticoagulation for the purpose of reducing the risk of ischemic stroke from AF may be even smaller than we observed. We also do not know whether a patient was non-compliant with prescribed warfarin or whether warfarin was not prescribed in the first place. Our data do not extend beyond 2005 to formally evaluate the impact of the KDOQI guidelines on warfarin use in dialysis patients with AF. Thus, the evidence gap persists on whether the benefits of warfarin outweigh its risks among dialysis patients with AF, and our study is not designed to fill it. We are unable to study aspirin use in this population, as it is available cheaply over the counter and won’t be registered as a claim. Our results may not be applicable to more affluent and younger Medicare patients as the dispensing data were only available in those with low to middle income enrolled in two regional state pharmacy benefit programs. Finally, the present study is based on data from 2 U.S. states, and its findings may not be generalizable to other parts of the U.S. or health care systems outside the U.S.
Our study mirrors findings from the general population indicating that older patients and non-whites were less likely to receive warfarin treatment.(20, 23) The reasons for these disparities are unclear but may relate to physician misperception of warfarin-related risks among these subgroups of patients.(18) We also confirmed the increasing prevalence of AF in older patients with hemodialysis, who bear a large burden of comorbidity and are at high risk of poor outcomes.(2)
Our study is novel in that it reports rather low warfarin use in hemodialysis patients with AF and absence of any temporal trends towards increased use.(24-25) In light of the increasing number of older dialysis patients with AF and the potentially catastrophic outcomes of either warfarin prophylaxis or omission of it, high research priority should be given to quality observational or randomized studies of this treatment indication in this vulnerable population.
ACKNOWLEDGMENTS
This work was supported by grant 1R21DK077336 from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health to Dr. Winkelmayer.
REFERENCES
- 1.Wizemann V, Tong L, Satayathum S, et al. Atrial fibrillation in hemodialysis patients: clinical features and associations with anticoagulant therapy. Kidney international. 2010;77:1098–106. doi: 10.1038/ki.2009.477. [DOI] [PubMed] [Google Scholar]
- 2.Winkelmayer WC, Patrick AR, Liu J, Brookhart MA, Setoguchi S. The increasing prevalence of atrial fibrillation among hemodialysis patients. J Am Soc Nephrol. 2011;22:357–65. doi: 10.1681/ASN.2010050459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seliger SL, Gillen DL, Longstreth WT, Jr., Kestenbaum B, Stehman-Breen CO. Elevated risk of stroke among patients with end-stage renal disease. Kidney international. 2003;64:603–9. doi: 10.1046/j.1523-1755.2003.00101.x. [DOI] [PubMed] [Google Scholar]
- 4.Fuster V, Ryden LE, Asinger RW, et al. ACC/AHA/ESC Guidelines for the Management of Patients With Atrial Fibrillation: Executive Summary A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients With Atrial Fibrillation) Developed in Collaboration With the North American Society of Pacing and Electrophysiology. Circulation. 2001;104:2118–50. [PubMed] [Google Scholar]
- 5.Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257–354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 6.Bennett WM. Should dialysis patients ever receive warfarin and for what reasons? Clin J Am Soc Nephrol. 2006;1:1357–9. doi: 10.2215/CJN.01700506. [DOI] [PubMed] [Google Scholar]
- 7.Vazquez E, Sanchez-Perales C, Garcia-Cortes MJ, et al. Ought dialysis patients with atrial fibrillation be treated with oral anticoagulants? Int J Cardiol. 2003;87:135–9. doi: 10.1016/s0167-5273(02)00317-0. discussion 9-41. [DOI] [PubMed] [Google Scholar]
- 8.Quinn RR, Naimark DM, Oliver MJ, Bayoumi AM. Should hemodialysis patients with atrial fibrillation undergo systemic anticoagulation? A cost-utility analysis. Am J Kidney Dis. 2007;50:421–32. doi: 10.1053/j.ajkd.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 9.Chan KE, Lazarus JM, Thadhani R, Hakim RM. Warfarin use associates with increased risk for stroke in hemodialysis patients with atrial fibrillation. J Am Soc Nephrol. 2009;20:2223–33. doi: 10.1681/ASN.2009030319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiesholzer M, Harm F, Tomasec G, Barbieri G, Putz D, Balcke P. Incidence of stroke among chronic hemodialysis patients with nonrheumatic atrial fibrillation. Am J Nephrol. 2001;21:35–9. doi: 10.1159/000046216. [DOI] [PubMed] [Google Scholar]
- 11.Wasse H, Gillen DL, Ball AM, et al. Risk factors for upper gastrointestinal bleeding among end-stage renal disease patients. Kidney international. 2003;64:1455–61. doi: 10.1046/j.1523-1755.2003.00225.x. [DOI] [PubMed] [Google Scholar]
- 12.Elliott MJ, Zimmerman D, Holden RM. Warfarin anticoagulation in hemodialysis patients: a systematic review of bleeding rates. Am J Kidney Dis. 2007;50:433–40. doi: 10.1053/j.ajkd.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 13.Fang MC, Stafford RS, Ruskin JN, Singer DE. National trends in antiarrhythmic and antithrombotic medication use in atrial fibrillation. Arch Intern Med. 2004;164:55–60. doi: 10.1001/archinte.164.1.55. [DOI] [PubMed] [Google Scholar]
- 14.Lakshminarayan K, Solid CA, Collins AJ, Anderson DC, Herzog CA. Atrial fibrillation and stroke in the general medicare population: a 10-year perspective (1992 to 2002). Stroke; a journal of cerebral circulation. 2006;37:1969–74. doi: 10.1161/01.STR.0000230607.07928.17. [DOI] [PubMed] [Google Scholar]
- 15.Yan Y, Birman-Deych E, Radford MJ, Nilasena DS, Gage BF. Comorbidity indices to predict mortality from Medicare data: results from the national registry of atrial fibrillation. Medical care. 2005;43:1073–7. doi: 10.1097/01.mlr.0000182477.29129.86. [DOI] [PubMed] [Google Scholar]
- 16.Kokotailo RA, Hill MD. Coding of stroke and stroke risk factors using international classification of diseases, revisions 9 and 10. Stroke; a journal of cerebral circulation. 2005;36:1776–81. doi: 10.1161/01.STR.0000174293.17959.a1. [DOI] [PubMed] [Google Scholar]
- 17.Lentine KL, Schnitzler MA, Abbott KC, Bramesfeld K, Buchanan PM, Brennan DC. Sensitivity of billing claims for cardiovascular disease events among kidney transplant recipients. Clin J Am Soc Nephrol. 2009;4:1213–21. doi: 10.2215/CJN.00670109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choudhry NK, Anderson GM, Laupacis A, Ross-Degnan D, Normand SL, Soumerai SB. Impact of adverse events on prescribing warfarin in patients with atrial fibrillation: matched pair analysis. Bmj. 2006;332:141–5. doi: 10.1136/bmj.38698.709572.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lentine KL, Schnitzler MA, Abbott KC, et al. Incidence, Predictors, and Associated Outcomes of Atrial Fibrillation after Kidney Transplantation. Clin J Am Soc Nephrol. 2006;1:288–96. doi: 10.2215/CJN.00920805. [DOI] [PubMed] [Google Scholar]
- 20.Choudhry NK, Soumerai SB, Normand SL, Ross-Degnan D, Laupacis A, Anderson GM. Warfarin prescribing in atrial fibrillation: the impact of physician, patient, and hospital characteristics. The American journal of medicine. 2006;119:607–15. doi: 10.1016/j.amjmed.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 21.KDOQI Clinical Practice Guidelines for Cardiovascular Disease in Dialysis Patients [1/5, 2011];Section I. Guidelines on evaluation and management of cardiovascular diseases. Guideline 9: Cerebrovascular Disease. 2005 (at http://www.kidney.org/professionals/KDOQI/guidelines_cvd/guide9.htm.)
- 22.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158:915–20. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 23.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–5. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 24.Wattigney WA, Mensah GA, Croft JB. Increasing trends in hospitalization for atrial fibrillation in the United States, 1985 through 1999: implications for primary prevention. Circulation. 2003;108:711–6. doi: 10.1161/01.CIR.0000083722.42033.0A. [DOI] [PubMed] [Google Scholar]
- 25.Khairallah F, Ezzedine R, Ganz LI, London B, Saba S. Epidemiology and determinants of outcome of admissions for atrial fibrillation in the United States from 1996 to 2001. Am J Cardiol. 2004;94:500–4. doi: 10.1016/j.amjcard.2004.04.068. [DOI] [PubMed] [Google Scholar]