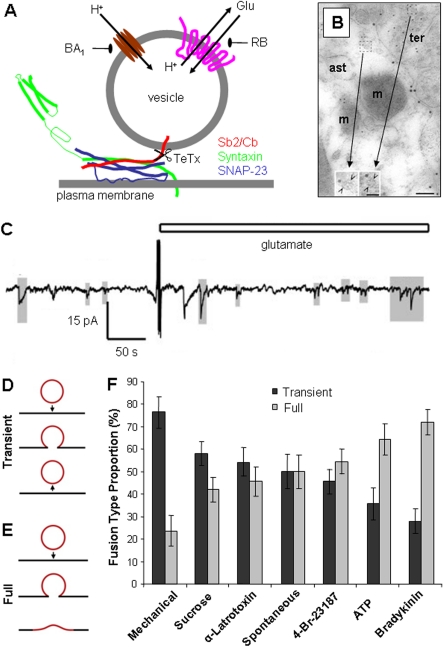
Figure 2. Release of gliotransmitters from astrocytes.
(A) Ca2+-dependent glutamate release from astrocytes utilizes a vesicular exocytotic pathway, which can be inhibited by: (i) a holoprotein of tetanus toxin (TeTx) that cleaves vesicular SNAREs Sb2 and cellubrevin (Cb, red); (ii) bafilomycin A1 (BA1) a specific inhibitor of V-ATPase (brown) which pumps protons into the vesicular lumen; and (ii) Rose Bengal (RB), an allosteric site modulator of VGLUTs (pink), transporters that utilize proton gradient to deliver glutamate (Glu) into the vesicle. The plasma membrane SNAREs syntaxin 1 (green) and SNAP-23 (blue) form an acceptor complex for the vesicular SNARE (Sb2/Cb red). Scissors and arrows indicate sites of actions for agents affecting vesicular proteins. Drawing is not to scale. (B) Glutamatergic vesicles at the putative tripartite synapse. Immunoelectron micrograph of VGLUT1 (small gold particles) in astrocytic process (ast) is identified by the plasma membrane glutamate transporters labelling (large gold particles). VGLUT1 positive vesicles (insets, open arrowheads) in an astrocyte and in the adjacent neuronal terminal (ter) have similar appearances; m, mitochondria. Scale bars, 100 nm; and 50 nm in insets. Reprinted by permission from Macmillan Publishers Ltd: Nature Neuroscience, Paola Bezzi, Vidar Gundersen, José Luis Galbete, Gerald Seifert, Christian Steinhäuser et al., 2004, Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate, Nature Neuroscience, 7(6), pp. 613−620, copyright (2004). (C) Exocytotic release of ATP from astrocytes is quantal. Small transient inward currents (shaded in grey), representing quantal release, can be recorded from ATP-sensing HEK-293 cells cultured on astrocytes at rest (spontaneous release) and after these glial cells were stimulated with glutamate (open bar); stimulation significantly decreases the mean interval between detected currents. Reproduced from Tina Pangršič, Maja Potokar, Matjaž Stenovec, Marko Kreft, Elsa Fabbretti, Andrea Nistri, Evgeny Pryazhnikov, Leonard Khiroug, Rashid Giniatullin, Robert Zorec, Exocytotic Release of ATP from Cultured Astrocytes, Journal of Biological Chemistry, 282(39), pp 28749–28758. Copyright © 2007, by the American Society for Biochemistry and Molecular Biology. (D–F) Preferred vesicular fusion type in astrocytes is stimulus dependent. (D, E) Drawings depict two vesicle fusion types described in astrocytes: (D) transient fusion, where a vesicle remains intact and opens a transient fusion pore (arrow pointing down, fusion; arrow pointing up, vesicle retrieval from the plasma membrane); and (E) full fusion where a vesicle fully collapses into the plasma membrane upon fusion (arrow). (F) Fusion events, categorized as either transient or full fusions were plotted as a percentage of the total number of fusions that occurred, either spontaneously or when cultured astrocytes were exposed to various stimuli. Reproduced from Erik B. Malarkey and Vladimir Parpura, Temporal characteristics of vesicular fusion in astrocytes: examination of synaptobrevin 2-laden vesicles at singlevesicle resolution, Journal of Physiology, John Wiley and Sons © 2011 The Authors. Journal compilation © 2011 The Physiological Society.