Figure 11.
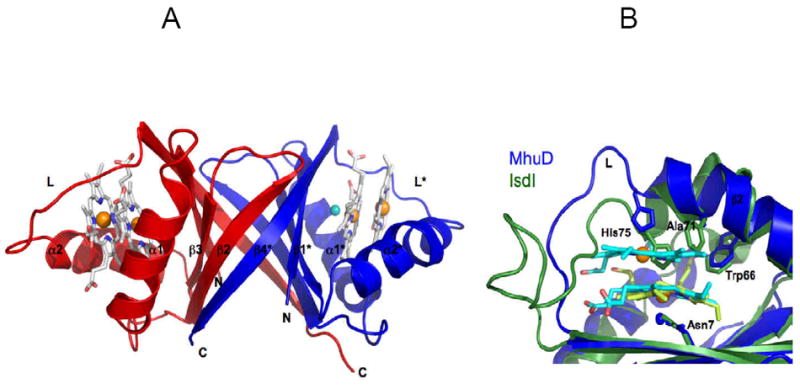
Cartoon representation of MhuD-diheme (PDB code 3HXP). (A) Each subunit in the dimer (chain A, blue; chain B, red) binds to two hemes, represented as sticks models with carbon (white), nitrogen (blue), and oxygen (red), as well as Fe (orange) and Cl- (cyan) spheres. (B) Heme binding sites of MhuD and Isd proteins. Superposition shows an extended α-helix 2 in MhuD (blue) which accommodates 2 hemes (cyan) while it is kinked by 45° in IsdI (green). The loop region, L, in MhuD makes extensive contacts to both hemes while that of IsdI is mostly extruded into solution. The side chains of the conserved Asn-His-Trp triad of residues, as well as Ala71 in MhuD and Phe72 in IsdI, are represented as stick models.
