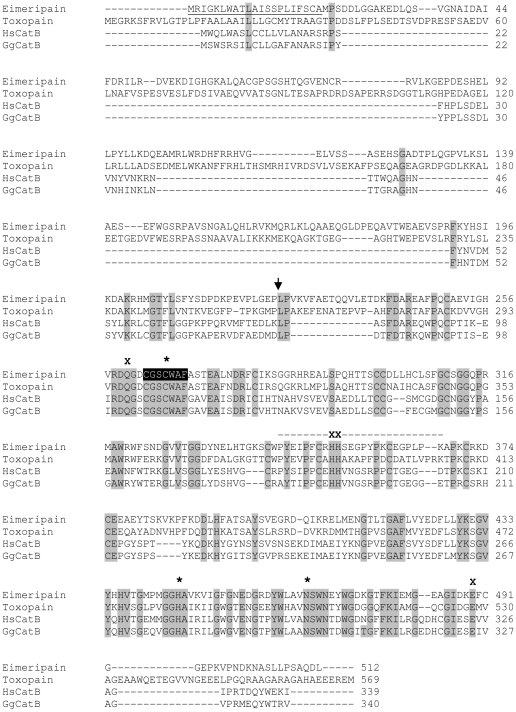
Figure 4. Sequence alignment of the pro-protein of the E. tenella cathepsin B.
An alignment of amino acid sequences of the E. tenella cathepsin B with cathepsin B expressed by T. gondii (toxopain-1, Accession n° AAL60053), Homo sapiens (HscatB, AAC37547.1) and Gallus gallus (GgCatB, AAA87075.1) is shown. The predicted signal peptide, according to SignalP (www.expasy.org) for the E. tenella cathepsin B is underlined. Identical residues are shaded in gray. The arrow indicates the probable cleavage site separating the pro- from the catalytic domain. Asterisks indicate the conserved essential catalytic triad residues. The predicted conserved occluding loop is shown with a dashed line. The conserved peptide sequence containing the catalytic Cys266 is highlighted in black. The characteristic aminoacids of cathepsin B like proteases are indicated by a cross and correspond to Gln260 of the oxyanion hole, the two His352-His353 of the occluding loop, and Glu489, at the base of the S2 pocket.