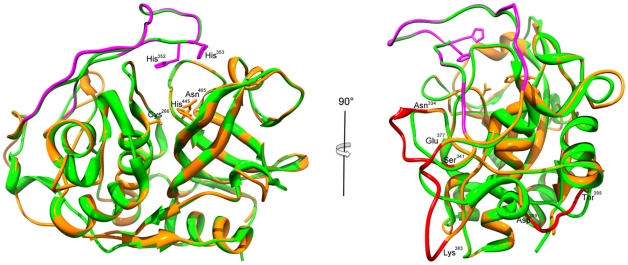
Figure 5. Ribbon diagram superposition of the catalytic domains of the E. tenella cathepsin B and human cathepsin B.
The X-ray crystallographic structure of the human cathepsin B (pdb 1 gmy) is colored in green and the proposed structure of homology-based model of the E. tenella cathepsin B is in orange. The catalytic triad residues (Cys266, His445 and Asn465) are depicted in the ball-and-stick representation. On the left panel, the occluding loop is represented in purple with the two adjacent histidine residues (His352, His353) in the ball-and-stick representation. On the right panel, the surface exposed loops specific to E. tenella cathepsin B are in red. The residues delimitating the loops: Asn334-Ser341, Glu377-Lys383 and Asp389-Thr395 are shown.