Figure 5. Super reactivity of Ser and Thr in peptide ASEHATYG when near to His.
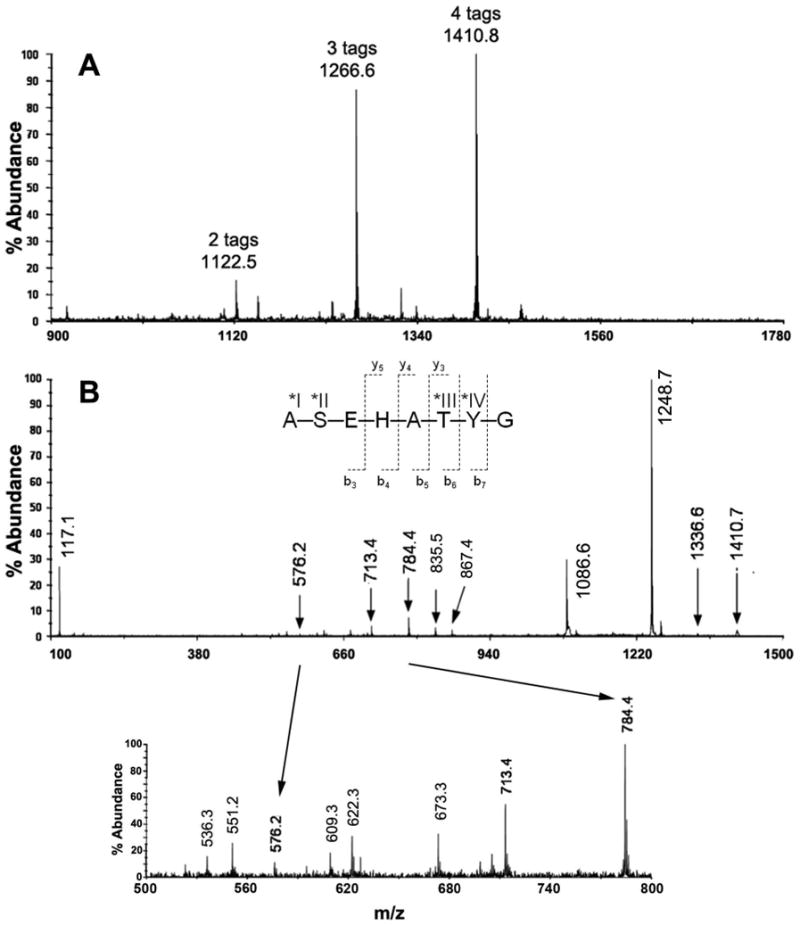
(A) MALDI MS spectrum of ASEHATYG from a single HPLC fraction, containing a mixture of peptide modified with two, three, and four iTRAQ groups at m/z 1122.5, 1266.6, and 1410.8, respectively. All four possible reactive sites—α-amine at N-terminal Ala (*I), and the hydroxyls on Ser (*II), Thr (*III), and Tyr (*IV) reacted with the iTRAQ reagent. (B) MS/MS spectrum of ASEHATYG with four iTRAQ tags. The insert is a zoom region from m/z 500-900. According to this MS/MS result, the remaining two iTRAQ tags were located primarily on the N-terminal α-amine Ala, and on the hydroxyl-group of Ser. Hydroxylamine reversed the O-acyl iTRAQ modification (results not shown).
