Abstract
Background/Objectives
Insufficient blood supply to the heart results in ischemic injury manifested clinically as myocardial infarction (MI). Following ischemia, inflammation is provoked and related to the clinical outcomes. A recent basic science study indicates that complement factor MASP-2 plays an important role in animal models of ischemia/reperfusion injury. We investigated the role of MASP-2 in human acute myocardial ischemia in two clinical settings: (1) Acute MI, and (2) Open heart surgery.
Methods
A total of 187 human subjects were enrolled in this study, including 50 healthy individuals, 27 patients who were diagnosed of coronary artery disease (CAD) but without acute MI, 29 patients with acute MI referred for coronary angiography, and 81 cardiac surgery patients with surgically-induced global heart ischemia. Circulating MASP-2 levels were measured by ELISA.
Results
MASP-2 levels in the peripheral circulation were significantly reduced in MI patients compared with those of healthy individuals or of CAD patients without acute MI. The hypothesis that MASP-2 was activated during acute myocardial ischemia was evaluated in cardiac patients undergoing surgically-induced global heart ischemia. MASP-2 was found to be significantly reduced in the coronary circulation of such patients, and the reduction of MASP-2 levels correlated independently with the increase of the myocardial necrosis marker, cardiac troponin I.
Conclusions
These results indicate an involvement of MASP-2 in ischemia-related necrotic myocardial injury in humans.
Keywords: MASP-2, myocardial ischemia, necrosis
Introduction
Prolonged regional myocardial ischemia manifests clinically as myocardial infarction (MI), with atherosclerosis of the coronary arteries being the most common cause. Global heart ischemia, which affects the entire organ, can occur during cardiac surgical procedures that employ aortic cross-clamping (AXCL).
Once myocardial ischemia has occurred, limiting the extent of tissue injury is critical. While minimizing the duration of ischemia is the first priority, evidence from basic and clinical research suggests that inhibition of the inflammation provoked by ischemia improves the outcome [1–3]. For example, several complement factors of the innate immune system have been associated with acute inflammation after ischemia [4–6], and complement inhibitors have been tested in preclinical studies [7–9]. However, limited positive results were obtained with the few inhibitors subjected to clinical trials [6]. A possible explanation is that downstream components in the complement pathway, e.g., C3 or C5, were targeted, leaving earlier activators unaffected. Activation of earlier complement factors may modulate other pathways such as coagulation cascades [10], influencing the outcomes of heart ischemia [11, 12].
The three pathways in the complement system, classical, alternative and lectin, converge to activate C3 which enters a common cascade resulting in the deposition of a membrane-attack-complex on targets and release of C3a and C5a, chemoattractants for inflammatory cells.
Specific initiators are involved in each pathway, i.e., mannan-binding lectin (MBL) and ficolins in the lectin pathway. MBL and ficolins circulate in complexes with one of three MBL-associated serine proteases (MASPs) [13, 14], MASP-2 being the major player. The MASP-2 is activated when MBL or ficolins bind to certain carbohydrate patterns on pathogens [15–17]. The activated MASP-2 then cleaves C4 and C2 to form the C3 convertase, C4b2a. MASP-1 alone is insufficient to activate lectin pathway, as it fails to cleave C4, but may facilitate MASP-2 activation through C2 [18, 19]. The activations of MASP-1 and MASP-2, which are regulated by C1-inhibitor [20–22], ultimately lead to activation of complement C3.
A recent basic science study indicates that MASP-2 plays an important role in animal models of ischemia/reperfusion injury, and inhibition of MASP-2 protects mice from tissue injury [23]. Whether MASP-2 plays a similar role in humans is still unknown.
In the current study, the involvement of MASP-2 in human acute myocardial ischemia was investigated in two clinical settings:
Acute MI: The levels of MASP-2 in the peripheral blood of acute MI patients were compared with those of healthy individuals or CAD patients without acute MI, which served as controls to document changes induced by causes others than acute MI.
Open heart surgery: Because in clinical MI the time and extent of ischemia precipitating events may vary greatly, a controlled form of global heart ischemia occurring in patients undergoing cardiac surgery was investigated. MASP-2 levels were determined in the coronary sinus blood sampled immediately before and after global heart ischemia produced by AXCL, thus directly monitoring the coronary circulation exiting the myocardium. In addition, peripheral blood samples were collected to document changes induced by aspects of the surgery other than AXCL. MASP-2 levels in the coronary and peripheral blood samples of each patient were analyzed for correlation with the levels of cardiac troponin I (cTnI), a specific myocardial necrosis marker, to determine whether MASP-2 levels were associated with the extent of cardiac injury.
Methods
Healthy individuals and patients
Institutional Review Board (IRB) protocols for the study were approved both at SUNY-Downstate Medical Center and Lutheran Medical Center.
. Fifty healthy individuals with no history of cardiovascular diseases or diabetes mellitus were included as controls (through the service of Bioreclamation Inc., Hicksville, NY) (Table 1).
. Twenty-seven patients who were diagnosed of CAD but without acute MI were selected in the study (both male and female; over 21 years old) (Table 2). These CAD patients were served as controls to document changes induced by causes others than acute MI. CAD on presentation was diagnosed by combining medical history, electrocardiogram, stress testing, echocardiography and coronary angiography. Patients with anemia, i.e., hemoglobin <7.0 mg/dl, were excluded. Plasma samples were collected and stored frozen at −80°C.
. Twenty-nine patients with acute MI referred for coronary angiography to the Cardiac Catheterization Laboratories at the two hospitals were selected in the study (both male and female; over 21 years old) (Table 3). Acute MI on presentation was diagnosed by combining a history of chest pain, and ST-segment elevation on ECG (STEMI)/or cTnI level ≥1.0 ng/ml for non-ST-segment elevation MI (NSTEMI). Patients with anemia, i.e., hemoglobin <7.0 mg/dl, were excluded. The average time from the onset of acute MI to the blood draw for this study was about 2 days. After centrifugation, plasma and blood cells were stored frozen at −80°C.
. A total of 81 adult patients undergoing elective open heart surgery with cardiopulmonary bypass (CPB) and aortic cross-clamping (AXCL) consented to be enrolled into the study. Procedures for myocardial protection during the surgery were carried out as previously described [24]. Coronary sinus blood samples were collected twice during surgery: a) 5 minutes after CPB had begun, prior to AXCL; b) within 5 minutes after the end of AXCL. In addition, peripheral blood samples were obtained at 7 pre-defined time points in the pre, intra, and post-operative periods1) pre-surgery/before skin incision; 2) after skin incision/prior to sternotomy; 3) 5 minutes after the start of CPB/before AXCL; 4) 5 minutes after cessation of AXCL; 5) 5 minutes after CPB cessation; 6) postoperative day 1; 7) postoperative day 2.
Table 1.
Demographic and baseline data of 50 normal controls
| Age | 40 (18–61) | |
| Gender (male %) | 86% | |
| Body mass index (kg/m2) | 28 (18–47) | |
| Smoker | 80% | |
| Diabetes Mellitus | 0 | |
| Cardiovascular diseases | 0 | |
| Race/Ethnics | African American | 48% |
| Caucasian | 40% | |
| Hispanic | 12% | |
Table 2.
Demographic and baseline data of 27 CAD patients without acute MI
| Age | 66 (43–92) | |
| Gender (male %) | 37% | |
| Body mass index (kg/m2) | 28 (21–37) | |
| Smoker | 25% | |
| Diabetes | 41% | |
| Hypercholesterolemia | 81% | |
| Hypertension | 96% | |
| Previous history of MI | 22% | |
| Race/Ethnics | African American | 89% |
| Caucasian | 7% | |
| Asian | 4% | |
Table 3.
Demographic and baseline data of 29 acute MI patients
| Age | 61 (32–72) | |
| Gender (male %) | 69% | |
| Body mass index (kg/m2) | 28 (13–43) | |
| Smoker | 41% | |
| Diabetes | 41% | |
| Hypercholesterolemia | 54% | |
| Hypertension | 76% | |
| Family history of cardiovascular diseases | 28% | |
| Diagnosis of STEMI | 28% | |
| NYHA classification (average) | 1.8 | |
| CCS classification (average) | 2.4 | |
| Race/Ethnics | African American | 59% |
| Caucasian | 10% | |
| Hispanic | 31% | |
Of the initial 81 patients, complete sets of coronary sinus and peripheral blood samples were obtained from 50 patients. In each of the remaining patients, who were not included in the study, at least 1 blood sample was missed due to the exigencies of cardiac surgery. A total of 450 blood samples were tested and included in the final analyses.
Relevant demographic parameters and the length of AXCL and CPB times for open heart surgery were recorded. cTnI levels were determined by the SUNY Downstate Clinical Pathology Laboratory on blood samples collected pre-surgery, immediately post-surgery, 8 hours post-surgery, and post-surgery days 1 and day 2.
Quantification of MASP-2
The plasma concentrations of MASP-2 in healthy and acute MI patients were measured by ELISA as described previously [25]. The plasma concentrations of MASP-2 in open heart surgery patients were measured by the ELISA kits supplied by Hycult, Netherlands, according to the manufacturer’s protocol. The kit used the same principles as the method used for the peripheral blood samples [25].
Statistical analysis
The plasma levels of MASP-2 from each patient and their relevant clinical parameters were entered into a Microsoft Excel database. All data were expressed as mean ± standard deviation (STEDV), and statistical analyses were performed using SPSS 18 Software (SPSS Inc., Chicago, IL). One-way ANOVA test was used to analyze the statistical differences of MASP2 levels among groups of acute MI patients, healthy individual, and CAD patients without acute MI. Post hoc Dunnett T3 test was used when the equality of variances was not met (Levene’s test was used to determine the homogeneity of variances). Paired t-test with two tails and unequal variances was used to analyze the statistical differences of MASP2 levels between time points of open heart surgery. Box-charts were plotted using SigmaPlot 10 software (Systat Software, Point Richmond, CA). Potential correlation between the levels of MASP-2 and the post-operation levels of cTnI were analyzed by Spearman’s Rho nonparametric correlation. Correlation analyses were performed with cTnI levels immediately after surgery, except variables at post-operation day 1 and 2 were correlated with the respective cTnI at post-operation day 1 or day 2. Mann-Whitney Test was used to compare cTnI levels in patients grouped by male versus female gender, diabetes mellitus versus non-diabetes, and current smokers versus non-smokers. The correlation of cardiac surgery types with cTnI was carried out by Kruskal-Walis Test. Multivariate regression analysis was used to test whether MASP-2 can independently predict post-operation cTnI increase. Post hoc power analyses were performed using G*Power 3.1 [26].
Results
MASP-2 levels in healthy individuals, CAD patients without acute MI and acute MI patients
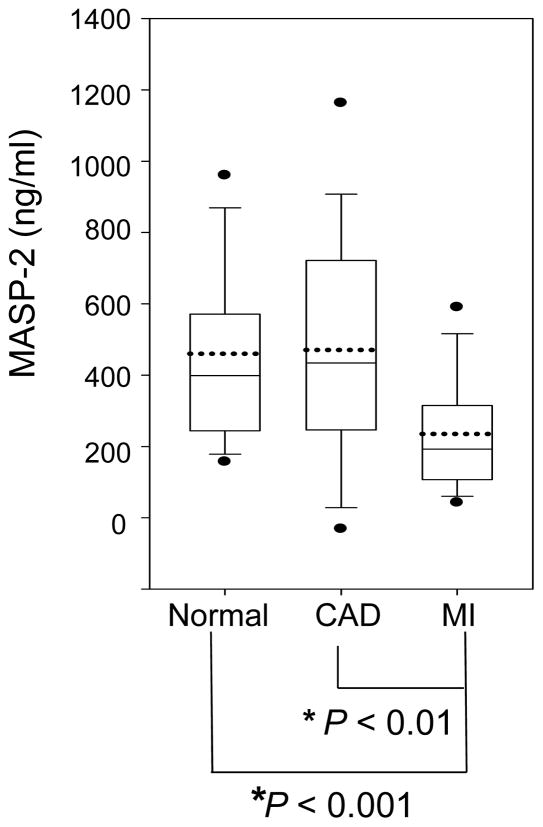
MASP-2 levels were significantly reduced about 50% in acute MI patients (235 ± 168 ng/ml) compared with healthy individuals (460 ± 259 ng/ml, P < 0.001) or CAD patients without acute MI (471 ± 327 ng/ml, P < 0.01) (Fig. 1).). The post hoc power analysis revealed 100% power for the detected difference between MI and healthy individuals, and 96% power for the detected difference between MI and CAD patients without acute MI. There was no statistical difference between the MASP-2 levels of healthy individuals and those of CAD patients without acute MI (P = 0.885).
Figure 1. MASP-2 in healthy individuals, CAD patients without acute MI and patients with acute MI.
MASP-2 levels were evaluated by ELISA in plasma samples from 50 healthy individuals, 27 CAD patients without acute MI and 29 acute MI patients. Statistical significances were analyzed as described in Methods. In the box-chart, the boundary of the box closest to zero indicates the 25th percentile, while the boundary of the box farthest from zero indicates the 75th percentile. Whiskers (error bars) above and below the box indicate the 90th and 10th percentiles. The two filled circles above and below the box indicate the 95th and 5th percentiles. The solid line within the box marks the median, and the dotted line marks mean (average). *indicates a statistical significance (P < 0.05).
MASP-2 levels in the coronary circulation during global heart ischemia in humans
To test if the reduction of MASP-2 was caused by activation during myocardial ischemia, we investigated the clinical scenario of the surgically-induced global heart ischemia which occurred due to AXCL in the normal course of open heart surgery (Fig. 2).
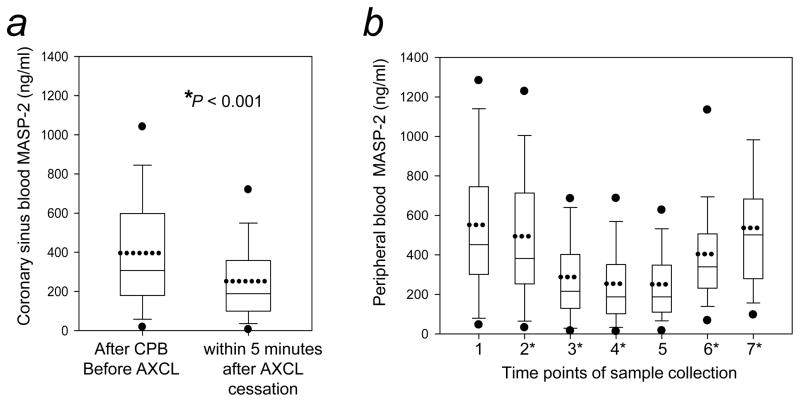
Figure 2. MASP-2 in the coronary and peripheral circulations during global heart ischemia induced by the aortic-cross clamping (AXCL) of open heart surgery.
MASP-2 levels were determined in blood samples taken from 50 patients undergoing open heart surgery. (a) MASP-2 levels in coronary circulation. Coronary sinus blood representing the circulation exiting the myocardium was collected before the start of AXCL and within 5 minutes after AXCL cessation. (b) MASP-2 levels in peripheral circulation. Peripheral blood samples were collected at the following pre-defined time points to document changes due to surgical procedures separate from the effect of AXCL on coronary circulation: 1) pre-surgery/before skin incision; 2) after skin incision/prior to sternotomy; 3) 5 minutes after the beginning of CPB; 4) 5 minutes after the end of AXCL; 5) 5 minutes after CPB cessation; 6) post-surgery day 1; 7) post-surgery day 2. Statistical significances were analyzed as described in Methods. * indicates statistical significance of the respective time point versus the previous time point (P<0.05). Box-charts were plotted as described in Figure 1.
Coronary sinus MASP-2 levels decreased by 40% during the period of AXCL (prior to AXCL = 396 ± 306 ng/ml, after AXCL cessation = 253 ± 209 ng/ml, P < 0.001) (Fig. 2a). The post hoc power analysis revealed 100% power for the detected difference. Note that, as is apparent when Figure 2b, time point 1, is compared with Figure 1, these patients had normal circulating MASP-2 levels prior to surgery (561 ± 389 ng/ml, P = 0.171).
MASP-2 levels were also significantly lower in the peripheral blood during the period of CPB that included AXCL, compared with levels prior to surgery (Fig. 2b, time points 2 and 3 compared with time point 1; Table 4). During the period of AXCL there was a small (12%) but significant reduction of MASP-2 in peripheral blood (prior to AXCL = 288 ± 230 ng/ml, after AXCL cessation = 254 ± 207 ng/ml, P < 0.01) (Fig. 2b, time points 3 and 4; Table 4). The post hoc power analysis revealed 98% power for the detected difference.
Table 4.
Univariate analysis correlating each of the listed variables with the post-surgery cTnI levels in 50 patients undergoing open heart surgery.§
| Variables | Mean ± SD | Correlation coefficient | P-value | |
|---|---|---|---|---|
| Age (year) | 64 ± 12 | 0.216 | 0.140 | |
| Male | 69% | 0.722 | ||
| Body mass index (kg/m2) | 28 ± 5 | 0.145 | 0.326 | |
| Left ventricle ejection fraction | 43 ± 19% | 0.038 | 0.843 | |
| Diabetes mellitus | 49% | 0.289 | ||
| Current Smokers | 27% | 0.189 | ||
| Cardiac Surgery Types: | ||||
| Coronary artery bypass grafting (CABG) | 38% |
|
||
| Valvular replacement | 32% | 0.191 | ||
| Combined CABG and valvular replacement | 30% | |||
| Cardiopulmonary bypass (CPB) time (min) | 119 ± 35 | 0.509 | 0.0002* | |
| Aortic cross clamping (AXCL) time (min) | 75 ± 31 | 0.383 | 0.007* | |
|
| ||||
| Coronary sinus MASP-2 levels (ng/ml): | ||||
| After CPB/before AXCL | 395 ± 303 | −0.218 | 0.137 | |
| 5 min. after AXCL cessation | 253 ± 207 | −0.291 | 0.045* | |
| Peripheral MASP-2 levels (ng/ml): | ||||
| 1. Pre-surgery/before skin incision | 561 ± 389 | −0.215 | 0.142 | |
| 2. After skin incision/Prior to sternotomy | 494 ± 367 | −0.236 | 0.106 | |
| 3. 5min after CPB start/before the start of AXCL | 288 ± 230 | −0.175 | 0.233 | |
| 4. Within 5min after AXCL cessation | 254 ± 206 | −0.265 | 0.069 | |
| 5. 5min after CPB cessation | 251 ± 196 | −0.257 | 0.078 | |
| 6. Post-surgery day 1 | 405 ± 274 | −0.139 | 0.335 | |
| 7. Post-surgery day 2 | 537 ± 346 | −0.159 | 0.298 | |
Spearman’s correlation analyses were carried out using the cTnI levels obtained immediately after surgery (post-surgery 0 hour), except for variables obtained at post-surgery days 1 and 2, which were correlated with the cTnI values obtained at post-surgery days 1 and 2, respectively. Mann-Whitney Test was used to compare cTnI levels in patients grouped by male versus female gender, diabetes mellitus versus non-diabetes, and current smokers versus non-smokers. The correlation of cardiac surgery types with cTnI levels was carried out by Kruskal-Walis Test.
Indicate statistical significance P < 0.05.
Correlation between coronary MASP-2 levels and the myocardial necrosis marker cTnI
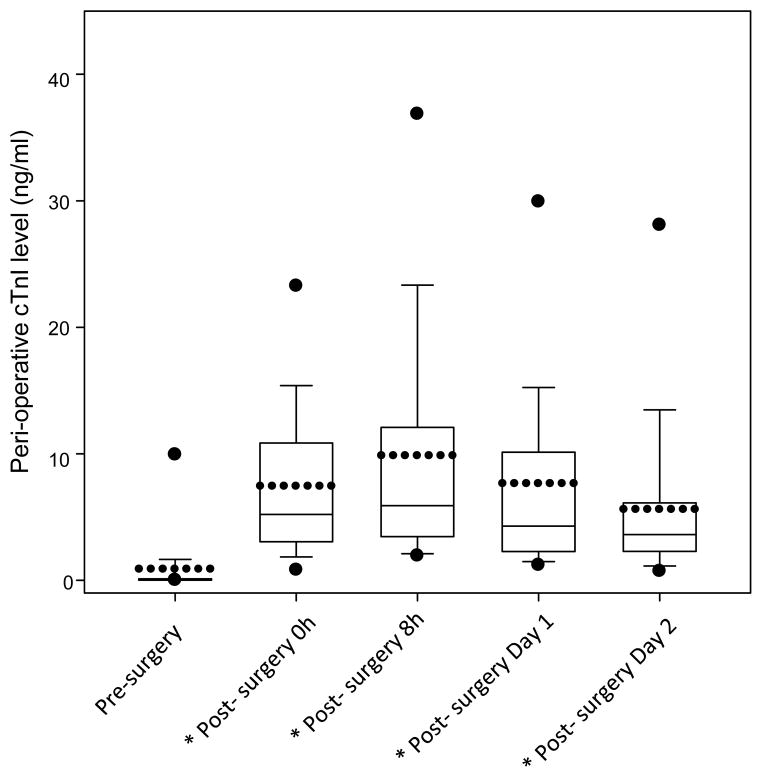
Previous clinical studies found that peripheral blood levels of the myocardial necrosis marker cTnI increased after open heart surgery and correlated with the length of time of AXCL and CPB [27, 28]. We confirmed that in our patients undergoing open heart surgery, cTnI levels increased significantly following surgery (P < 0.001, paired t-test comparing cTnI levels in each post-surgery time points with that of the pre-surgery time point) (Fig. 3).
Figure 3. cTnI, a specific cardiac necrosis marker, was significantly increased for an extended period after open heart surgery.
cTnI levels in the peripheral blood were measured by ELISA in samples collected before surgery, immediately after surgery, and on post-surgery day 1 and 2. Box-chart was plotted as described in Figure 1. *indicates a statistical significance.
Univariate analysis showed that among the relevant demographic and surgical variables, the length of CPB and AXCL times correlated significantly with immediate post-surgery cTnI levels (Table 4). Among MASP-2 levels tested at different time points, peripheral or coronary circulations, only coronary MASP-2 levels 5 minutes after AXCL cessation correlated inversely with post-surgery 0 hour cTnI levels (Spearman correlation coefficient = −0.291, P = 0.045) (Table 4). The post hoc power analysis revealed 98% power for the detected correlation.
To test if the coronary MASP-2 level after AXCL cessation was independently associated with cTnI elevation, a multivariate analysis controlling for CPB and AXCL times was carried out (Table 5). After adjusting for these variables, the coronary MASP-2 level was found to be an independent predictor for the elevated cTnI level at post-surgery 0 hour (P = 0.026 when the CPB time was entered into the multivariate model 1; P = 0.025 when the AXCL time was entered into the multivariate model 2).
Table 5.
Multivariate analysis of independent predictors for post-operational cTnI levels in patients with open heart surgery.
| Variable | P-Value |
|---|---|
| Model 1: | |
| MASP-2 (coronary; 5min post-AXCL) | 0.026* |
| CPB time | 0.0003* |
|
| |
| Model 2: | |
| MASP-2 (coronary; 5min post-AXCL) | 0.025* |
| AXCL time | 0.005* |
Discussion
MASP-2, a mediator of complement activation via the lectin pathway, was significantly reduced in the peripheral blood in patients with acute MI compared with healthy individuals or CAD patients without acute MI (Fig. 1).
The hypothesis, that MASP-2 was activated following myocardial ischemia, was tested in patients whose hearts experienced controlled global ischemia produced by the surgical clamping of the aorta during open heart surgery. The global ischemia reduced MASP-2 in the coronary circulation by 40%, a reduction much greater than the 12% occurring in the peripheral circulation during the time that the heart was subjected to AXCL (Fig. 2). The greater reduction may reflect the major difference between the coronary and the peripheral blood, which was caused by the cardiac arrest during cardiopulmonary bypass to the heart. Nevertheless, changes of MASP-2 levels in both coronary sinus and systemic circulation also reflect the systemic activation of the inflammatory system induced by cardiopulmonary bypass, in which ischemia-reperfusion is a co-player together with blood contact with artificial surface, cardiotomy suction, endotoxin release and other stimuli.
An alternative hypothesis may also explain the lower MASP-2 levels in acute MI patients: MASP-2 levels were low in these patients prior to the development of acute MI, e.g. genetically defective through single nucleotide polymorphism (SNP) polymorphism. Certain SNP in MASP2 gene has been shown to lower MASP-2 protein concentration or result in a dysfunctional protein [29]. Future studies will help to address the potential involvement of MASP-2 gene polymorphism in acute MI.
The coronary levels of post-AXCL MASP-2 correlated inversely and independently with the post-surgery increase of myocardial necrosis marker, cTnI, indicating a potential contribution of MASP-2 to necrotic cardiac injury following ischemia. To our knowledge, this is the first report implicating MASP-2 in ischemic injury in a clinical setting. Our independent findings in humans are in agreement with those of a recent animal study [23], which shows that either a MASP-2 deficiency or an anti-MASP-2 Ab protects mice against injury in both a heart and an intestinal ischemia/reperfusion model.
The independent effects of other determinants and MASP-2 on post-operative cTnI levels were also evaluated. The correlation between coronary post-AXCL MASP-2 levels and post-operative cTnI levels was found independent of gender, smoking and BMI. However, the MASP-2 and cTnI correlation was dependent of age and surgical types. It is possible that aging and types of surgical trauma may affect MASP-2 expression and the susceptibility of myocardium to ischemia.
Clinical studies of complement in ischemic injury have generally been carried out on samples from peripheral blood. Complement factor C3, a downstream factor of MASP-2, was reported to be activated in peripheral circulation in patients undergoing open heart surgery with CPB [30, 31], and was associated with pathogenic post-operation conditions [32–36]. It has been reported that post-operative peak cTnI >13ng/ml was an independent predictor of short and mid-term clinical outcome after cardiac surgery [37]. We analyzed the subgroup of patients with post-operative peak cTnI > 13ng/ml and found that their coronary post-AXCL MASP-2 levels were significantly correlated with peak cTnI levels (P = 0.011). The rest of patients with post-operative peak cTnI < 13ng/ml did not show significant correlation between coronary post-AXCL MASP-2 levels and peak cTnI levels (P > 0.05). Thus, it is possible that MASP-2 levels are associated with pathogenic post-operation conditions, and future follow up studies will help to determine such a correlation.
In the present study, we analyzed samples from both the coronary and peripheral blood. We were able to distinguished between the effects of a systemic activation of complement in blood (as a result of general surgical procedures/action of the heart-lung bypass machine) versus global myocardial ischemia. Our results suggest a possible mechanism in myocardial ischemia, that either regional (i.e. MI) or global (e.g. AXCL-induced in cardiac surgery) ischemia, could expose certain self-targets to circulating lectin complement factors. The recognition of self-targets by lectins in turn activates MASP-2, subsequently causing it to be depleted from the circulation through the action of C1-inhibitor.
Interestingly, C1-inhibitor in the coronary sinus blood of our cardiac patients had a small but significant decrease after AXCL (pre-AXCL = 394 ± 51 μg/ml, post-AXCL = 374 ± 53 μg/ml, P = 0.01; data not shown). On the other hand, C1-inhibitor in peripheral blood did not have significant change immediately before or after AXCL (270 ± 53 μg/ml vs. 276 ± 42 μg/ml, respectively, P = 0.291; data not shown). These results support our hypothesis that MASP-2 was activated in heart ischemia and subsequently removed by C1-inhibitor.
The role of the lectin pathway in ischemia-related conditions has been investigated in animal models. Studies in mice indicated that a deficiency of MBL, an upstream factor of MASP-2, protected against ischemia/reperfusion injury [38, 39]. However, clinical studies of MBL in ischemic heart diseases, including MI, have generated conflicting reports. Some found that the level of MBL was inversely associated with the risk of MI [40] and the outcome after MI [41] or stroke [42]. A study of peripheral blood in open heart surgery showed that MBL levels were decreased at 30 and 240 minutes after CPB compared with preoperative levels, suggesting its involvement in post-operative inflammation [43]. Others reported that MBL levels were initially normal in patients with post-MI heart failure, but were decreased at a 1 month follow-up, and that the latter may be inversely associated with a higher incidence of re-infarction [44]. Diverging observations were also reported. High blood levels of MBL were reported to be associated with increased risk of ischemic heart disease, myocardial infarction, and premature death [45–47]. One clinical study showed that MBL-deficiency was not associated with MI [48]. Further studies are needed to define the role of MBL in the clinical development of MI or in long term recovery after MI.
In summary, we conclude that MASP-2 activation is involved in ischemia-related necrotic myocardial injury in humans.
Acknowledgments
The authors would like to thank Dr. James Cottrell for his continued support through Brooklyn Anesthesia Research, Inc., Michael Lee, Amy Gleed, Hsiao-ying Chin, Donna Newman, Chris Johnson, and the staff of the Downstate Cardiac Catheterization Laboratory and of the Cardiothoracic-Intensive Care Unit for their valuable assistance. We also thank James Nelson, Kell Julliard, Drs. Robert Zaloom and Claudia Lyon for assistance with the IRB protocol and sample collection at Lutheran Medical Center. Dr. Jeremy Weedon at SUNY Downstate provided valuable assistance for biostatistics analysis. The authors greatly appreciate Dr. Julie Rushbrook for critical comments and thorough editing of the manuscript. The research was funded in part by NIH grant 1R21HL088527 (MZ), ECRIP Award from New York State-Department of Health (KS and MZ) and SUNY Downstate Dean’s Award for Pilot Project (MZ). Dr. Yunfang Hou is an ECRIP fellow.
Abbreviations
- MASP
MBL-associated serine proteases
- CPB
cardiopulmonary bypass
- AXCL
aortic cross-clamping
- cTnI
cardiac troponin I
- SNP
single nucleotide polymorphism
Footnotes
Disclosures: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Turer AT, Hill JA. Pathogenesis of myocardial ischemia-reperfusion injury and rationale for therapy. Am J Cardiol. 2010;106:360–8. doi: 10.1016/j.amjcard.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steffens S, Montecucco F, Mach F. The inflammatory response as a target to reduce myocardial ischaemia and reperfusion injury. Thromb Haemost. 2009;102:240–7. doi: 10.1160/TH08-12-0837. [DOI] [PubMed] [Google Scholar]
- 3.Napoli C, Lerman LO, de Nigris F, Gossl M, Balestrieri ML, Lerman A. Rethinking primary prevention of atherosclerosis-related diseases. Circulation. 2006;114:2517–27. doi: 10.1161/CIRCULATIONAHA.105.570358. [DOI] [PubMed] [Google Scholar]
- 4.Palikhe A, Sinisalo J, Seppanen M, Haario H, Meri S, Valtonen V, et al. Serum complement C3/C4 ratio, a novel marker for recurrent cardiovascular events. Am J Cardiol. 2007;99:890–5. doi: 10.1016/j.amjcard.2006.11.034. [DOI] [PubMed] [Google Scholar]
- 5.Celik T, Iyisoy A, Yuksel UC, Jata B, Ozkan M. The impact of admission C-reactive protein levels on the development of no-reflow phenomenon after primary PCI in patients with acute myocardial infarction: the role of inflammation. Int J Cardiol. 2009;136:86–8. doi: 10.1016/j.ijcard.2008.03.058. author reply 8–9. [DOI] [PubMed] [Google Scholar]
- 6.Cannon RO., 3rd Mechanisms, management and future directions for reperfusion injury after acute myocardial infarction. Nat Clin Pract Cardiovasc Med. 2005;2:88–94. doi: 10.1038/ncpcardio0096. [DOI] [PubMed] [Google Scholar]
- 7.Weisman HF, Bartow T, Leppo MK, Marsh HC, Jr, Carson GR, Concino MF, et al. Soluble human complement receptor type 1: in vivo inhibitor of complement suppressing post-ischemic myocardial inflammation and necrosis. Science. 1990;249:146–51. doi: 10.1126/science.2371562. [DOI] [PubMed] [Google Scholar]
- 8.Vakeva AP, Agah A, Rollins SA, Matis LA, Li L, Stahl GL. Myocardial infarction and apoptosis after myocardial ischemia and reperfusion: role of the terminal complement components and inhibition by anti-C5 therapy. Circulation. 1998;97:2259–67. doi: 10.1161/01.cir.97.22.2259. [DOI] [PubMed] [Google Scholar]
- 9.Undar A, Eichstaedt HC, Clubb FJ, Jr, Fung M, Lu M, Bigley JE, et al. Novel anti-factor D monoclonal antibody inhibits complement and leukocyte activation in a baboon model of cardiopulmonary bypass. Ann Thorac Surg. 2002;74:355–62. doi: 10.1016/s0003-4975(02)03656-1. discussion 62. [DOI] [PubMed] [Google Scholar]
- 10.Amara U, Rittirsch D, Flierl M, Bruckner U, Klos A, Gebhard F, et al. Interaction between the coagulation and complement system. Adv Exp Med Biol. 2008;632:71–9. doi: 10.1007/978-0-387-78952-1_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spiess BD. Ischemia--a coagulation problem? J Cardiovasc Pharmacol. 1996;27 (Suppl 1):S38–41. doi: 10.1097/00005344-199600001-00009. [DOI] [PubMed] [Google Scholar]
- 12.Tracy RP, Kleiman NS, Thompson B, Cannon CP, Bovill EG, Brown RG, et al. Relation of coagulation parameters to patency and recurrent ischemia in the Thrombolysis in Myocardial Infarction (TIMI) Phase II Trial. Am Heart J. 1998;135:29–37. doi: 10.1016/s0002-8703(98)70339-4. [DOI] [PubMed] [Google Scholar]
- 13.Matsushita M, Fujita T. Activation of the classical complement pathway by mannose-binding protein in association with a novel C1s-like serine protease. J Exp Med. 1992;176:1497–502. doi: 10.1084/jem.176.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwaeble W, Dahl MR, Thiel S, Stover C, Jensenius JC. The mannan-binding lectin-associated serine proteases (MASPs) and MAp19: four components of the lectin pathway activation complex encoded by two genes. Immunobiology. 2002;205:455–66. doi: 10.1078/0171-2985-00146. [DOI] [PubMed] [Google Scholar]
- 15.Roos A, Bouwman LH, Munoz J, Zuiverloon T, Faber-Krol MC, Fallaux-van den Houten FC, et al. Functional characterization of the lectin pathway of complement in human serum. Mol Immunol. 2003;39:655–68. doi: 10.1016/s0161-5890(02)00254-7. [DOI] [PubMed] [Google Scholar]
- 16.Turner MW. The role of mannose-binding lectin in health and disease. Mol Immunol. 2003;40:423–9. doi: 10.1016/s0161-5890(03)00155-x. [DOI] [PubMed] [Google Scholar]
- 17.Tsutsumi A, Takahashi R, Sumida T. Mannose binding lectin: genetics and autoimmune disease. Autoimmun Rev. 2005;4:364–72. doi: 10.1016/j.autrev.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Matsushita M, Thiel S, Jensenius JC, Terai I, Fujita T. Proteolytic activities of two types of mannose-binding lectin-associated serine protease. J Immunol. 2000;165:2637–42. doi: 10.4049/jimmunol.165.5.2637. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi M, Iwaki D, Kanno K, Ishida Y, Xiong J, Matsushita M, et al. Mannose-binding lectin (MBL)-associated serine protease (MASP)-1 contributes to activation of the lectin complement pathway. J Immunol. 2008;180:6132–8. doi: 10.4049/jimmunol.180.9.6132. [DOI] [PubMed] [Google Scholar]
- 20.Petersen SV, Thiel S, Jensen L, Vorup-Jensen T, Koch C, Jensenius JC. Control of the classical and the MBL pathway of complement activation. Mol Immunol. 2000;37:803–11. doi: 10.1016/s0161-5890(01)00004-9. [DOI] [PubMed] [Google Scholar]
- 21.Presanis JS, Hajela K, Ambrus G, Gal P, Sim RB. Differential substrate and inhibitor profiles for human MASP-1 and MASP-2. Mol Immunol. 2004;40:921–9. doi: 10.1016/j.molimm.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Kerr FK, Thomas AR, Wijeyewickrema LC, Whisstock JC, Boyd SE, Kaiserman D, et al. Elucidation of the substrate specificity of the MASP-2 protease of the lectin complement pathway and identification of the enzyme as a major physiological target of the serpin, C1-inhibitor. Mol Immunol. 2008;45:670–7. doi: 10.1016/j.molimm.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Schwaeble WJ, Lynch NJ, Clark JE, Marber M, Samani NJ, Ali YM, et al. Targeting of mannan-binding lectin-associated serine protease-2 confers protection from myocardial and gastrointestinal ischemia/reperfusion injury. Proc Natl Acad Sci U S A. 2011;108:7523–8. doi: 10.1073/pnas.1101748108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosengart TK, Finnin EB, Kim DY, Samy SA, Tanhehco Y, Ko W, et al. Open heart surgery in the elderly: results from a consecutive series of 100 patients aged 85 years or older. Am J Med. 2002;112:143–7. doi: 10.1016/s0002-9343(01)01097-x. [DOI] [PubMed] [Google Scholar]
- 25.Moller-Kristensen M, Jensenius JC, Jensen L, Thielens N, Rossi V, Arlaud G, et al. Levels of mannan-binding lectin-associated serine protease-2 in healthy individuals. J Immunol Methods. 2003;282:159–67. doi: 10.1016/j.jim.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149–60. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 27.Vermes E, Mesguich M, Houel R, Soustelle C, Le Besnerais P, Hillion ML, et al. Cardiac troponin I release after open heart surgery: a marker of myocardial protection? Ann Thorac Surg. 2000;70:2087–90. doi: 10.1016/s0003-4975(00)02152-4. [DOI] [PubMed] [Google Scholar]
- 28.Greenson N, Macoviak J, Krishnaswamy P, Morrisey R, James C, Clopton P, et al. Usefulness of cardiac troponin I in patients undergoing open heart surgery. Am Heart J. 2001;141:447–55. doi: 10.1067/mhj.2001.113071. [DOI] [PubMed] [Google Scholar]
- 29.Thiel S, Steffensen R, Christensen IJ, Ip WK, Lau YL, Reason IJ, et al. Deficiency of mannan-binding lectin associated serine protease-2 due to missense polymorphisms. Genes Immun. 2007;8:154–63. doi: 10.1038/sj.gene.6364373. [DOI] [PubMed] [Google Scholar]
- 30.Semb AG, Vaage J, Sorlie D, Lie M, Mjos OD. Coronary trapping of a complement activation product (C3a des-Arg) during myocardial reperfusion in open-heart surgery. Scand J Thorac Cardiovasc Surg. 1990;24:223–7. doi: 10.3109/14017439009098073. [DOI] [PubMed] [Google Scholar]
- 31.Pekna M, Nilsson L, Nilsson-Ekdahl K, Nilsson UR, Nilsson B. Evidence for iC3 generation during cardiopulmonary bypass as the result of blood-gas interaction. Clin Exp Immunol. 1993;91:404–9. doi: 10.1111/j.1365-2249.1993.tb05916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meri S, Verkkala K, Miettinen A, Valtonen V, Linder E. Complement levels and C3 breakdown products in open-heart surgery: association of C3 conversion with the postpericardiotomy syndrome. Clin Exp Immunol. 1985;60:597–604. [PMC free article] [PubMed] [Google Scholar]
- 33.Seghaye MC, Duchateau J, Grabitz RG, Faymonville ML, Messmer BJ, Buro-Rathsmann K, et al. Complement activation during cardiopulmonary bypass in infants and children. Relation to postoperative multiple system organ failure. J Thorac Cardiovasc Surg. 1993;106:978–87. [PubMed] [Google Scholar]
- 34.Segal H, Sheikh S, Kallis P, Cottam S, Beard C, Potter D, et al. Complement activation during major surgery: the effect of extracorporeal circuits and high-dose aprotinin. J Cardiothorac Vasc Anesth. 1998;12:542–7. doi: 10.1016/s1053-0770(98)90098-2. [DOI] [PubMed] [Google Scholar]
- 35.Tarnok A, Hambsch J, Emmrich F, Sack U, van Son J, Bellinghausen W, et al. Complement activation, cytokines, and adhesion molecules in children undergoing cardiac surgery with or without cardiopulmonary bypass. Pediatr Cardiol. 1999;20:113–25. doi: 10.1007/s002469900417. [DOI] [PubMed] [Google Scholar]
- 36.Bruins P, te Velthuis H, Yazdanbakhsh AP, Jansen PG, van Hardevelt FW, de Beaumont EM, et al. Activation of the complement system during and after cardiopulmonary bypass surgery: postsurgery activation involves C-reactive protein and is associated with postoperative arrhythmia. Circulation. 1997;96:3542–8. doi: 10.1161/01.cir.96.10.3542. [DOI] [PubMed] [Google Scholar]
- 37.Paparella D, Cappabianca G, Visicchio G, Galeone A, Marzovillo A, Gallo N, et al. Cardiac troponin I release after coronary artery bypass grafting operation: effects on operative and midterm survival. Ann Thorac Surg. 2005;80:1758–64. doi: 10.1016/j.athoracsur.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 38.Walsh MC, Bourcier T, Takahashi K, Shi L, Busche MN, Rother RP, et al. Mannose-binding lectin is a regulator of inflammation that accompanies myocardial ischemia and reperfusion injury. J Immunol. 2005;175:541–6. doi: 10.4049/jimmunol.175.1.541. [DOI] [PubMed] [Google Scholar]
- 39.Zhang M, Takahashi K, Alicot EM, Vorup-Jensen T, Kessler B, Thiel S, et al. Activation of the Lectin Pathway by Natural IgM in a Model of Ischemia/Reperfusion Injury. J Immunol. 2006;177:4727–34. doi: 10.4049/jimmunol.177.7.4727. [DOI] [PubMed] [Google Scholar]
- 40.Saevarsdottir S, Oskarsson OO, Aspelund T, Eiriksdottir G, Vikingsdottir T, Gudnason V, et al. Mannan binding lectin as an adjunct to risk assessment for myocardial infarction in individuals with enhanced risk. J Exp Med. 2005;201:117–25. doi: 10.1084/jem.20041431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trendelenburg M, Theroux P, Stebbins A, Granger C, Armstrong P, Pfisterer M. Influence of functional deficiency of complement mannose-binding lectin on outcome of patients with acute ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Eur Heart J. 2010;31:1181–7. doi: 10.1093/eurheartj/ehp597. [DOI] [PubMed] [Google Scholar]
- 42.Cervera A, Planas AM, Justicia C, Urra X, Jensenius JC, Torres F, et al. Genetically-defined deficiency of mannose-binding lectin is associated with protection after experimental stroke in mice and outcome in human stroke. PLoS One. 2010;5:e8433. doi: 10.1371/journal.pone.0008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marcheix B, Carrier M, Martel C, Cossette M, Pellerin M, Bouchard D, et al. Effect of pericardial blood processing on postoperative inflammation and the complement pathways. Ann Thorac Surg. 2008;85:530–5. doi: 10.1016/j.athoracsur.2007.08.050. [DOI] [PubMed] [Google Scholar]
- 44.Ueland T, Espevik T, Kjekshus J, Gullestad L, Omland T, Squire IB, et al. Mannose binding lectin and soluble Toll-like receptor 2 in heart failure following acute myocardial infarction. J Card Fail. 2006;12:659–63. doi: 10.1016/j.cardfail.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 45.Troelsen LN, Garred P, Madsen HO, Jacobsen S. Genetically determined high serum levels of mannose-binding lectin and agalactosyl IgG are associated with ischemic heart disease in rheumatoid arthritis. Arthritis Rheum. 2007;56:21–9. doi: 10.1002/art.22302. [DOI] [PubMed] [Google Scholar]
- 46.Haahr-Pedersen S, Bjerre M, Flyvbjerg A, Mogelvang R, Dominquez H, Hansen TK, et al. Level of complement activity predicts cardiac dysfunction after acute myocardial infarction treated with primary percutaneous coronary intervention. J Invasive Cardiol. 2009;21:13–9. [PubMed] [Google Scholar]
- 47.Pesonen E, Hallman M, Sarna S, Andsberg E, Haataja R, Meri S, et al. Mannose-binding lectin as a risk factor for acute coronary syndromes. Ann Med. 2009;41:591–8. doi: 10.1080/07853890903110994. [DOI] [PubMed] [Google Scholar]
- 48.Calvo-Alen J, Alarcon GS, Tew MB, Tan FK, McGwin G, Jr, Fessler BJ, et al. Systemic lupus erythematosus in a multiethnic US cohort: XXXIV. Deficient mannose-binding lectin exon 1 polymorphisms are associated with cerebrovascular but not with other arterial thrombotic events. Arthritis Rheum. 2006;54:1940–5. doi: 10.1002/art.21787. [DOI] [PubMed] [Google Scholar]
- 49.Endo Y, Matsushita M, Fujita T. Role of ficolin in innate immunity and its molecular basis. Immunobiology. 2007;212:371–9. doi: 10.1016/j.imbio.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 50.Garred P, Honore C, Ma YJ, Munthe-Fog L, Hummelshoj T. MBL2, FCN1, FCN2 and FCN3-The genes behind the initiation of the lectin pathway of complement. Mol Immunol. 2009;46:2737–44. doi: 10.1016/j.molimm.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 51.Runza VL, Schwaeble W, Mannel DN. Ficolins: novel pattern recognition molecules of the innate immune response. Immunobiology. 2008;213:297–306. doi: 10.1016/j.imbio.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 52.Krarup A, Sorensen UB, Matsushita M, Jensenius JC, Thiel S. Effect of capsulation of opportunistic pathogenic bacteria on binding of the pattern recognition molecules mannan-binding lectin, L-ficolin, and H-ficolin. Infect Immun. 2005;73:1052–60. doi: 10.1128/IAI.73.2.1052-1060.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuraya M, Ming Z, Liu X, Matsushita M, Fujita T. Specific binding of L-ficolin and H-ficolin to apoptotic cells leads to complement activation. Immunobiology. 2005;209:689–97. doi: 10.1016/j.imbio.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 54.Jensen ML, Honore C, Hummelshoj T, Hansen BE, Madsen HO, Garred P. Ficolin-2 recognizes DNA and participates in the clearance of dying host cells. Mol Immunol. 2007;44:856–65. doi: 10.1016/j.molimm.2006.04.002. [DOI] [PubMed] [Google Scholar]