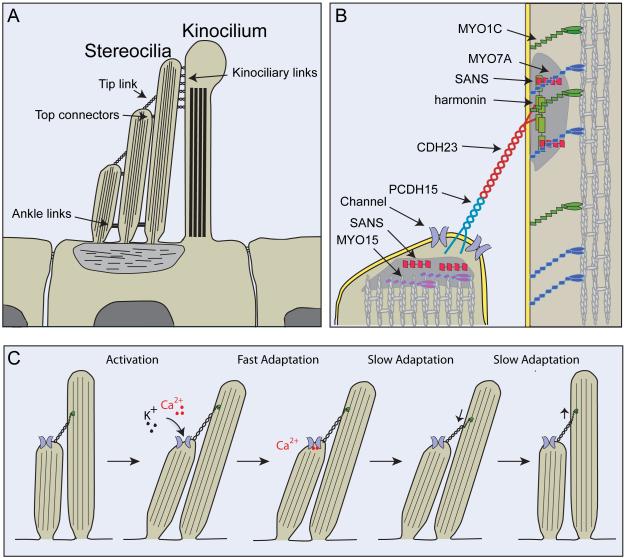
Fig. 2. Hair cells and their mechanotransduction machinery.
(A) Cross section through the apical part of a hair cell. Hair bundles consist of several rows of actin-rich stereocilia and a microtubule-based kinocilium. The sterocilia are connected to each other and to the kinocilium by extracellular filaments that can be visualized by electron microscopy. These are the tip links, top connectors, ankle links and kinociliary links (for a recent review see [4]). Note that the kinocilium, kinociliary links, ankle links and top connectors are present in murine cochlear hair cells only during hair bundle development. These structures degenerate once hair bundles have reached their mature shape and only stereocilia, tip links and top connectors remain [114]. (B) Diagram of the tip-link region, indicating molecules that are part of the tip-link complex. CDH23 homodimers form the upper part of the tip link and PCDH15 homodimers the lower part [59]. Two electron dense regions (shaded in gray) can be visualized by transmission electron microscopy in proximity to the upper and lower insertion sites of tip links [103]. Immunolocalization studies have localized the indicated proteins to the electron dense regions (for a recent review see [4]). (C) Current model of activation and adaptation of transduction channels in hair cells. Transduction channels that are located in proximity to the lower insertion site of tip links are opened by deflection of the hair bundle in the direction of the longest sterocilia. The tip link is thought to gate the channel. Ca2+ that flows into the stereocilia leads to fast adaptation likely by binding to the channel or a molecule nearby. Slow adaptation is thought to be regulated by a myosin motor complex at the upper insertion site of tip links. Upon Ca2+ entry, the adaptation motor is released from the cytoskeleton and slips down the actin filaments, leading to channel closure. Tension in the transduction complex is restored by movement of the myosin motor towards the tips of stereocilia (for a recent review see [1]). However, the localization of the transduction channel raises questions regarding models of slow adaptation because it places the site of Ca2+ entry into stereocilia at the lower tip-link end, far away from the proposed localization of the adaptation motor at the upper tip-link end. It has been proposed that Ca2+ entering through the transduction channel might affect the adaptation motor hooked up to the next tip link lower down in the same stereocilium [1], but adaptation motors in the longest stereocilia would then likely not show Ca2+-dependent adaptation.