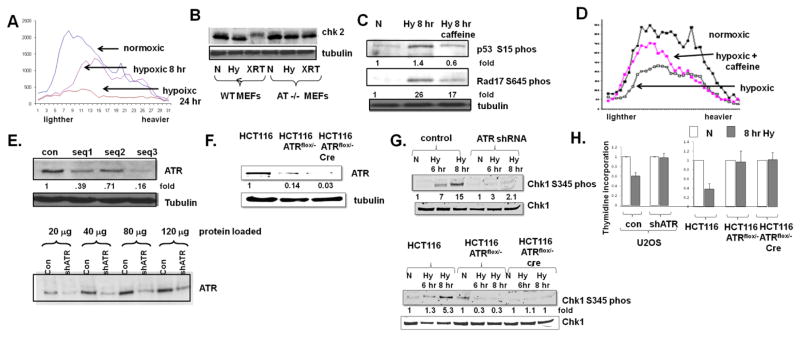
Fig 1.
ATR inhibits the initiation of DNA replication in hypoxic cells. A. U2OS cells were rendered hypoxic for 8 hrs or 24 hrs, and pulsed with thymidine prior to the collection of DNA and fractionation as described in the text. Lighter fractions represent smaller, newly synthesized DNA molecules. B. Chk2 expression in normoxic (N), hypoxic (Hy) and 10 Gy irradiated (XRT) MEFs and, as control, AT−/− MEFs. Phosphorylation of Chk2 manifests as a mobility shift. C. Expression of Chk1-serine 345 phosphorylation and rad17-serine 645 phosphorylation in cells rendered hypoxic and also rendered hypoxic and treated with 10 mM caffeine. D. Incoroporation of thymidine into DNA in cells rendered hypoxic for eight hours, with and without 10 mM caffeine. Experiments were replicated twice; fold changes are displayed. E. ATR expression in U2OS cells expressing three different shRNA sequences (top) and extent of ATR depletion as indicated by increasing loading of proteins (bottom). F. ATR expression in wild-type HCT116 cells, HCT116 ATRflox/− cells expressing a tamoxifen inducible cre recombinase, and HCT116 ATRflox/− cells treated with tamoxifen for 24 hrs. G. Chk1-serine 345 phosphorylation in cells described in (F) rendered hypoxic. H. Thymidine incorporation in U2OS and HCT116 depleted cells rendered hypoxic for eight hours. Experiments were replicated three times. Average ± standard error is displayed.