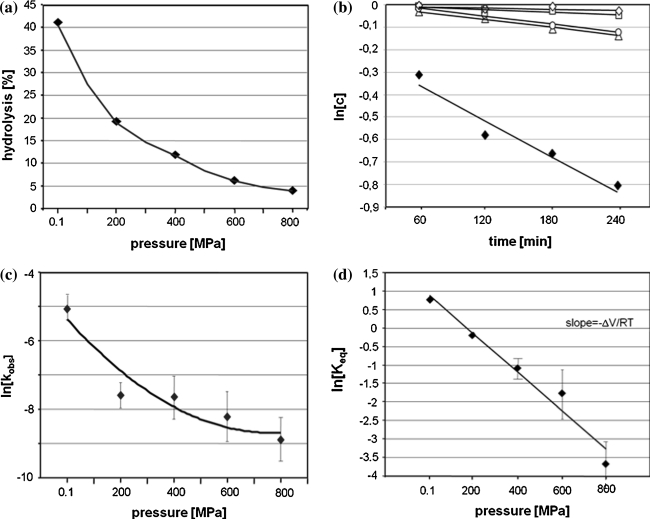
Fig. 2.
HHP dependence of yeast tRNAPhe cleavage induced with Pb2+. a Hydrolysis of tRNAPhe with Pb(II) dependent on pressure carried out for 2 h. b Determination of kobs by plotting the natural logarithm of product concentration at pressure: 0.1 MPa (filled diamonds). 200 MPa (open triangle), 400 MPa (open circle), 600 MPa (open square), 800 MPa (open diamonds) as a function of time. c Determination of the activation volume changes associated to the hydrolysis reaction. The ΔV≠ was calculated from the plot by curve fitting to equation: lnkobs = lnk0 − ΔV≠P/RT + Δβ≠P2/2RT. d Linear decrease of the theoretical equilibrium constants logarithms with increasing hydrostatic pressures. Keq was calculated as the ratio of cleaved and uncleaved fractions at equilibrium. The percentages of cleaved products were obtained from the extrapolation of each exponential. Change of the reaction volume (ΔV) was calculated from the slope of the curve RTlnKeq/pressure