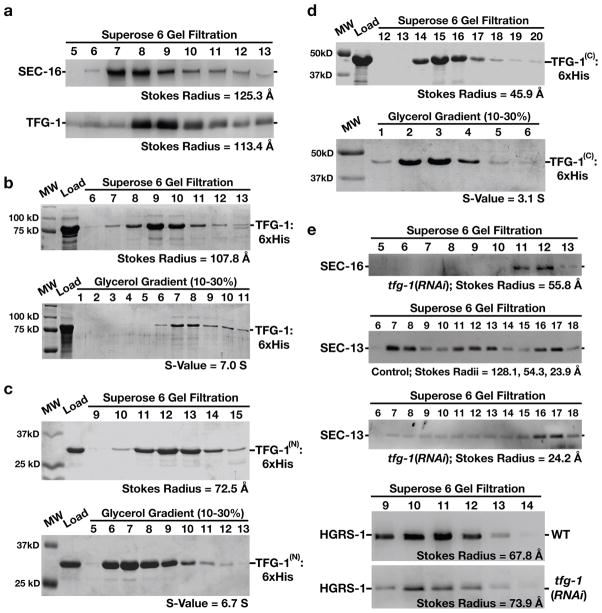
Figure 4. The amino-terminus of TFG-1 mediates it oligomerization.
The results presented in each panel are representative of at least three individual experiments performed. In all cases, the intensities of each band were measured to identify the peak elution fraction, which was used to calculate either a Stokes radius or sedimentation value, depending on the experiment. (a) Western blots using SEC-16 antibodies (top) or TFG-1 antibodies (bottom) of C. elegans embryo extract fractionated on a Superose 6 gel filtration column. The peaks corresponding to SEC-16 and TFG-1 partially overlap. A Stokes radius was calculated for each protein based on comparison with the elution profiles of known standards. (b-d) Recombinant polyhistidine-tagged TFG-1 or fragments of TFG-1 described in Figure 1e were expressed and purified from E. coli extracts using nickel resin. A Coomassie stained gel of the peak elution fractions after fractionation of the recombinant proteins on a Superose 6 gel filtration column are shown (top). Proteins were fractionated on a 10–30% glycerol gradient (bottom), and S-values were calculated based on the location of characterized standards run on a parallel gradient. (e) Western blots of control and TFG-1 depleted C. elegans whole worm extracts fractionated on a Superose 6 gel filtration column and probed with SEC-16 antibodies (top) or SEC-13 antibodies (middle panels). Fractionation of HGRS-1, a component of the ESCRT-0 complex, was examined in both control and TFG-1 depleted conditions (bottom panels), to ensure gel filtration profiles were directly comparable. Stokes radii were calculated for each protein based on comparison with the elution profiles of known standards.