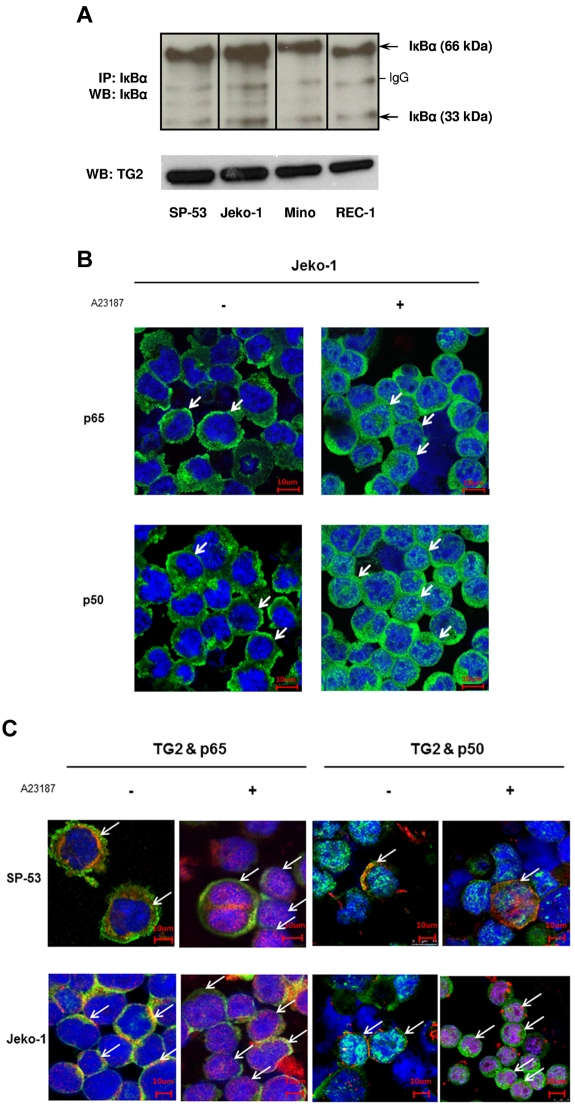
Figure 2.
TG2 is associated with NF-κB expression in MCL. (A) Cell lysates from 4 different MCL cell lines, SP-53, Jeko-1, Mino, and REC-1, were immunoprecipitated and immunoblotted with an anti-IκBα Ab. Analysis indicated the presence of strong dimeric forms (66 kDa) and monomeric forms (33 kDa) of IκBα. The polymeric forms of IκBα persisted in TG2-expressed MCL cells. IP indicates immunoprecipitation; WB, Western blot. (B) TG2 activation induced NF-κB activation after translocation to the nucleus and increased the expression of NF-κB components in MCL cells. SP-53 and Jeko-1 cells were treated with A23187 (2μM for 24 hours), followed by immunochemical staining using anti-p65 and anti-p50 Abs. Confocal microscopic images revealed the translocation of NF-κB components p65 and p50 into the nucleus (arrows) in response to A23187 treatment. A further increase in the cytoplasmic intensity of p65 and p50 was clearly observed in Jeko-1 cells after A23187 treatment. (C) TG2 and NF-κB components were colocalized in MCL cells. Confocal microscopic images showed the colocalization of TG2 and NF-κB components p65 and p50 (arrows). Before treatment with A23187, TG2 (red) and the NF-κB components p65 or p50 (green) were located mainly in the cytoplasm. After A23187 treatment (2μM for 24 hours), simultaneous translocations of TG2 and the NF-κB components p65 or p50 were observed.