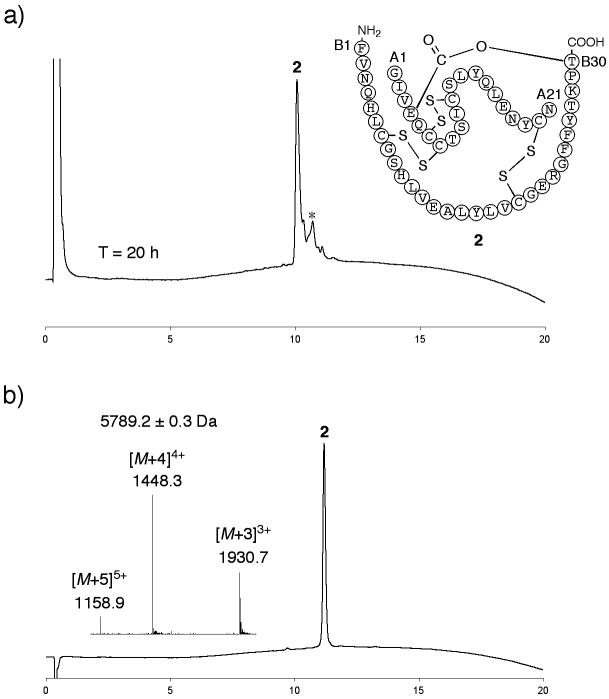
Figure 2. Folding/disulfides formation to give ester insulin.
a) Folding of the ester insulin precursor 1 to form ester insulin 2 was monitored by LC after 20 h (UV profiles at 214 nm are shown). Essentially similar data were obtained at T = 1 h. Folding conditions were 1: ∼0.3 mg mL−1, Tris: 20 mM, Cys: 8 mM, cystine: 1 mM, GnHCl: 1.5 M, pH = 7.3, *Cys adducts; b) purified ester insulin 2. (Inset) On-line ESI-MS spectra taken at the top of the main peak in chromatogram. The chromatographic separations were performed using a linear gradient (5-65%) of buffer B in buffer A over 15 min (buffer A = 0.1% TFA in water; buffer B = 0.08% TFA in acetonitrile) (UV profiles at 214 nm). Columns with different reverse phase packings were used for a) and b).