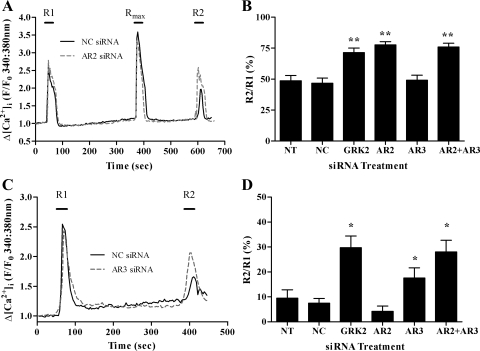
Fig. 4.
Arrestin depletion differentially attenuates the desensitization of UTP- and ET1-stimulated Ca2+ signaling. ASMCs were either nontransfected or nucleofected with negative control, anti-GRK2, anti-arrestin2, anti-arrestin3, or anti-arrestin2 + anti-arrestin 3 siRNAs. After 48 h, ASMCs were loaded with the Ca2+-sensitive dye Fura2-AM for 1 h before application of standard desensitization protocols. For UTP, ASMCs were challenged with approximate EC50 concentrations of UTP (10 μM) for 30 s before (R1) and after (R2) addition of a maximal UTP concentration (Rmax: 100 μM, for 30 s). For R1/R2 for endothelin type A (ETA) receptor desensitization, a maximal ET1 concentration (50 nM) was applied for 30 s before (termed R1) and after (termed R2) a 5-min washout period. Reduced R2/R1 ratios are interpreted as an indication of receptor desensitization. Representative traces are shown for UTP (A) or ET1 (C)-treated cells transfected with NC or AR2 siRNAs (A) or NC or AR3 siRNAs (C). Desensitization of agonist-stimulated Ca2+ signaling was determined as the relative change in R2 response compared with R1. Cumulative data are shown for UTP (B) and ET1 (D) as means ± SE for % changes in R2 relative to R1; 20–38 cells for each treatment, from ≥4 experiments using cell preparations from ≥4 different animals. Statistically significant differences are indicated: *P < 0.05, **P < 0.01 vs. NC siRNA treatment (one-way ANOVA; Dunnett's post hoc test).