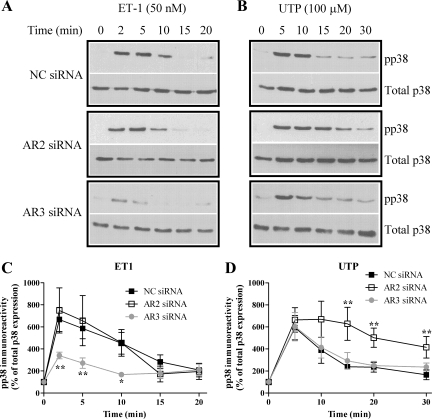
Fig. 6.
Arrestins differentially regulate ET1- and UTP-stimulated p38 MAPK signaling. ASMCs were nucleofected with 100 nM negative control, anti-arrestin2, or anti-arrestin3 siRNAs. After 24 h, cells were serum starved for a further 24 h and then stimulated with ET1 (50 nM) or UTP (100 μM). A and B: ASMCs were lysed, and phospho-p38 MAPK levels were determined by standard immunoblotting techniques (top panels). To verify equal gel loading, all blots were stripped and reprobed with anti-p38 antibody (bottom panels). Representative immunoblots show the effects of isoform-specific arrestin depletion on ET1 (A)- or UTP (B)-stimulated phospho-p38 MAPK signals. C and D: cumulative densitometric analysis of ET1 (C)- or UTP (D)-stimulated p38 phosphorylation is shown; data are means ± SE for 4 experiments in cells prepared from 4 different animals. Arrestin3 depletion significantly attenuated ET1-stimulated phospho-p38 MAPK signals, while arrestin2 depletion significantly prolonged UTP-stimulated phospho-p38 MAPK signals when compared with cells transfected with NC siRNA (*P < 0.05, **P < 0.01; two-way ANOVA; Bonferroni's post hoc test).