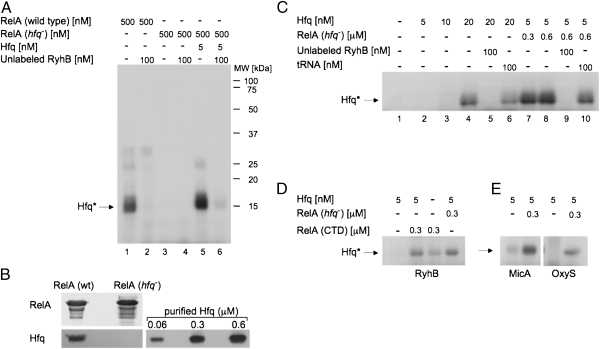
Fig. 3.
RelA stimulates binding of Hfq to RyhB in vitro. (A) RyhB RNA binds Hfq in RelA preparations. UV cross-linking of in vitro-synthesized 32P-labeled RyhB incubated with RelA protein purified from wild-type or hfq− cells. Where indicated, Hfq and/or unlabeled RyhB were added to the incubation mixtures. Proteins covalently bound to residues of labeled RNA were detected in SDS/PAGE (after boiling). Hfq protein bound to residues of labeled RyhB (Hfq*). (B) Detection of Hfq protein in samples of purified RelA. Twenty micrograms of RelA protein purified from wild-type or hfq mutant and samples of purified Hfq were separated by 15% SDS/PAGE after boiling in loading buffer. The upper part of the gel was stained with Coomassie blue dye, and the lower part of the same gel was analyzed using α-Hfq (Western blotting). (C) RelA stimulates RyhB binding by otherwise ineffective amounts of Hfq. UV cross-linking of labeled RyhB incubated with different concentrations of Hfq in the presence or absence of RelA purified from hfq− cells. Where indicated, unlabeled RyhB or yeast tRNA was added. (D) The C-terminal domain (CTD) of RelA is sufficient in stimulating RyhB binding by Hfq. UV cross-linking of labeled RyhB incubated with Hfq in the absence or the presence of RelA-CTD or RelA purified from hfq− cells. (E) RelA stimulates Hfq binding of unrelated RNAs as well. UV cross-linking labeled MicA and OxyS RNAs incubated with Hfq in the presence or the absence of RelA purified from hfq− cells. The proteins were detected as in A.