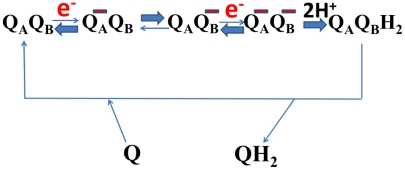
Fig. 2.
Proposed oxidoreduction function of two ubiquinone molecules in complex I. QA is the ubiquinone proposed to be bound near iron-sulfur cluster N2, whereas QB is another ubiquinone bound more weakly near the membrane domain (see Fig. 1). QA is insulated from protonation and its one-electron reduction is thermodynamically unfavorable (see text). Uptake of the second electron to yield the doubly negatively charged pair is again unfavorable. The doubly charged ubiquinone pair is proposed to drive conformational changes in the LMN subunit triad (see Fig. 3 A and B), which are reversed on protonation of QB. Reduced QB then exchanges with the membrane pool for oxidized Q.