Abstract
Our previous data showed the inhibitory effect of ethanol on AMP-activated protein kinase phosphorylation, which appears to be mediated, in part, through increased levels of hepatic ceramide and activation of protein phosphatase 2A (Liangpunsakul S, Sozio MS, Shin E, Zhao Z, Xu Y, Ross RA, Zeng Y, Crabb DW. Am J Physiol Gastrointest Liver Physiol 298: G1004–G1012, 2010). The effect of ethanol on AMP-activated protein kinase phosphorylation was reversed by imipramine, suggesting that the generation of ceramide via acid sphingomyelinase (ASMase) is stimulated by ethanol. In this study, we determined the effects of imipramine on the development of hepatic steatosis, the generation of ceramide, and downstream effects of ceramide on inflammatory, insulin, and apoptotic signaling pathways, in ethanol-fed mice. The effect of ethanol and imipramine (10 μg/g body wt ip) on ceramide levels, as well as inflammatory, insulin, and apoptotic signaling pathways, was studied in C57BL/6J mice fed the Lieber-DeCarli diet. Ethanol-fed mice developed the expected steatosis, and cotreatment with imipramine for the last 2 wk of ethanol feeding resulted in improvement in hepatic steatosis. Ethanol feeding for 4 wk induced impaired glucose tolerance compared with controls, and this was modestly improved with imipramine treatment. There was a significant decrease in total ceramide concentrations in response to imipramine in ethanol-fed mice treated with and without imipramine (287 ± 11 vs. 348 ± 12 pmol/mg tissue). The magnitude and specificity of inhibition on each ceramide species differed. A significant decrease was observed for C16 (28 ± 3 vs. 33 ± 2 pmol/mg tissue) and C24 (164 ± 9 vs. 201 ± 4 pmol/mg tissue) ceramide. Ethanol feeding increased the levels of the phosphorylated forms of ERK slightly and increased phospho-p38 and phospho-JNK substantially. The levels of phospho-p38 and phospho-JNK were reduced by treatment with imipramine. The activation of ASMase and generation of ceramide in response to ethanol feeding may underlie several effects of ethanol. ASMase inhibitors may be considered as a therapeutic target for alcohol-induced hepatic steatosis and activation of stress kinases.
ethanol treatment of hepatoma cells and mice inhibited the activity and lowered the protein level of AMP-activated protein kinase (AMPK), a central regulator of metabolism (38). This process leads to a decrease in fatty acid oxidation and increase in fatty acid synthesis. The mechanism of ethanol inhibition of AMPK is complex. Central to the control of AMPK activity is phosphorylation on Thr172, which is absolutely required for activity. Ethanol inhibits the upstream kinases for AMPK (such as PKC-ζ and LKB1) when they are stimulated by oxidative stress (17), thus reducing the phosphorylation and activity of AMPK. Ethanol also activates protein phosphatase 2A (PP2A) (18). PP2A belongs to a family of trimeric serine/threonine phosphatases controlling many cellular functions and signaling pathways, including apoptosis, insulin signaling, and the Wnt/β-catenin pathways. PP2A is involved in the regulation of many cellular functions and signaling pathways (12, 33). Our laboratory reported that PP2A-C subunit co-immunoprecipitated with AMPK (17). We also found that ethanol increased PP2A activity by ∼30% in hepatoma cells treated with ethanol at 50 mM for 24 h (16). We showed that the PP2A inhibitor okadaic acid or PP2A small interfering RNA significantly attenuated the inhibitory effect of ethanol on AMPK phosphorylation. Our results implied that ethanol-induced AMPK inhibition in hepatoma cells is partly mediated through the activation of PP2A. PP2A can be activated by ceramide (33), which was reported to bind to the B subunit; hence a form of PP2A was identified as a “ceramide-activated protein phosphatase” (18). Ceramide can be synthesized by the hydrolysis of sphingomyelin by sphingomyelinases (SMases), of which the acidic and neutral isoforms are of major relevance in the cells. Ceramide can also be synthesized in vivo in the endoplasmic reticulum, starting with the condensation of serine and palmitoyl-CoA, catalyzed by serine palmitoyl transferase. Ceramide is further metabolized by ceramidase to sphingosine, which can be converted back to ceramide by (dihydro)ceramide synthase (8), an enzyme that participates in the de novo pathway. Ethanol treatment of hepatoma cells significantly increased cellular (C16 and C18) ceramide content by ∼20% and increased PP2A activity by 18–23% (16). To differentiate which pathways might be involved in the action of ethanol, we tested the following inhibitors for their ability to block the effect of ethanol on AMPK activation: myriocin (an inhibitor of serine-palmitoyl transferase), GW4869 [an inhibitor of neutral SMase (NSMase)], fumonisin B1 [an inhibitor of (dihydro)ceramide synthase], and imipramine [an inhibitor of acid SMase (ASMase)]. We found that the myriocin and GW4869 did not interfere with the ability of ethanol to inhibit AMPK phosphorylation. However, the effect of ethanol on AMPK phosphorylation was reversed by imipramine, suggesting that the generation of ceramide via ASMase is stimulated by ethanol. This effect of ethanol on ceramide levels has been observed in mice fed ethanol for 4 wk: we found 28 and 36% increases in hepatic C16 ceramide and C18 ceramide, respectively (16). The level of ASMase mRNA, but not NSMase or serine palmitoyl transferase, was increased by 1.7-fold in ethanol-fed liver compared with controls. These results suggested that the effect of ethanol on AMPK in vivo might be mediated by the increased ceramide level, and that inhibition of ASMase could abrogate the ethanol-induced steatosis.
Chronic ethanol feeding is also reported to cause insulin resistance in the liver and whole animal (20). Wand's group has demonstrated that ethanol feeding impairs the ability of insulin to activate its intracellular signaling cascade, with a marked decrease in the activation of Akt (20). In other models of fatty liver, such as diet-induced obesity, experimental diabetes, and feeding of the methionine-choline-deficient diet, insulin resistance is also a prominent feature (25). Of interest, several lines of evidence have linked generation of ceramide in liver with insulin resistance (10), suggesting a novel mechanism for ethanol-induced insulin resistance. We, therefore, determined the effects of imipramine on the development of hepatic steatosis and insulin resistance in ethanol-fed mice, the generation of ceramide, and downstream effects of ceramide on inflammatory, insulin, and apoptotic signaling pathways.
METHODS
Animals and diets.
Six- to eight-week-old male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) were fed the Lieber-DeCarli diet, as previously described. In brief, animals were housed individually in a room with controlled temperature (20–22°C), humidity (55–65%), and lighting (on at 6 AM and off at 6 PM). Protein content was constant at 18% of calories, and each diet had identical mineral and vitamin content. The animals were divided into two dietary groups: 1) control diet (fat comprising 10% of total calories, 6% from cocoa butter, and 4% from safflower oil, 72% of calories as carbohydrate); and 2) ethanol-containing diet [identical to the control diet, but with ethanol added to account for 27.5% of total calories and the caloric equivalent of carbohydrate (maltose-dextrin) removed]. After 2 wk of these diets, the animals were subdivided into four experimental groups: 1) ethanol-containing diet (27.5% of total calories); 2) pair-fed control diet in which ethanol was replaced isocalorically with carbohydrate; 3) control diet with imipramine; and 4) ethanol-containing diet with imipramine. In the latter two groups, imipramine (Sigma, St. Louis, MO, catalog no. I0899) was administered intraperitoneally for the last 2 wk of the study. The drug was dissolved in saline and daily injected in a dosage of 10 μg/g body wt. The dose of the drug used (10 μg/g) was selected on the basis of doses used in previous studies (2, 22). Moreover, higher doses of imipramine (20 or 40 mg·kg−1·day−1) led to significant adverse effects, including reduction of food intake and significant body weight loss (28, 34). For animals on an ethanol-containing diet, cages were placed on heating pads to maintain body temperature, because ethanol consumption can induce hypothermia. After 4 wk of liquid diet feeding, the animals were killed, at which time blood and liver tissue were collected. The studies were approved by the Indiana University School of Medicine Animal Care and Use Committee and the Veterans Affairs Animal Use Subcommittee.
Analysis of ceramide in hepatic tissues.
At the time of death, liver tissues were harvested as rapidly as possible, immediately freeze-clamped with Wollenberger tongs at the temperature of liquid nitrogen, powdered under liquid nitrogen with a mortar and pestle, and stored at −80°C for analysis. Ten milligrams of liver tissue powder prepared under liquid nitrogen were used for the measurement of intracellular ceramide and sphingomyelin contents with mass spectrometry (MS). In brief, hepatic tissues were diluted in PBS (×1) to 0.5 ml, followed by addition of 3 ml of MeOH-chloroform (2:1) and the addition of C17–0 ceramide as an internal standard. The samples were vortexed for 1 min and incubated on ice for 10 min. One milliliter of chloroform and 1.3 ml of H2O were added to separate the phases. Then the samples were vortexed for 1 min and centrifuged (1,750 g for 10 min), and the lower phase was transferred to a new glass tube. Two milliliters of chloroform were added to the remaining upper phase left in the original tube to further extract the lipids. The lower phase was then transferred to the same tube, and the solvent was evaporated under nitrogen at room temperature. After evaporation of the solvent, the dried samples were dissolved in 100 μl of methanol; and 10 μl of sample were used for MS analyses. MS analyses were performed using API-4000 (Applied Biosystems/MDS SCIEX, Forster City, CA) with the Analyst data acquisition system. The instrument is equipped with a Z-spray ionization source. Both the nebulizer and desolvation gases are nitrogen, and the collision gas is argon. Typical operating parameters are as follows: nebulizing gas, 15; curtain gas, 8; collision-activated dissociation gas, 35; electrospray voltage 5,000 with positive-ion multiple reaction monitoring (MRM) mode; and a temperature of heater at 500°C. Precursor scan and MRM mode were used for measurement of ceramides. The ions monitored (in negative mode) were at mass-to-charge ratio of 550.2 (the parent ion): 294.3 (the product ion) for 17:0-Cer, 536.4:280.1 for 16:0-Cer, and 564.3:308.1 for 18:0-Cer. The dwell time in the MRM mode was 75 ms. Samples (10 μl) were loaded through a LC system (Agilent 1100) with an autosampler. The mobile phase was methanol-water-NH4OH (90:10:0.1 vol/vol/vol). The flow rate was 0.2 ml/min and 1.5 min/sample.
Hepatic histology, and triglyceride, cytokine, and apoptosis analysis.
A part of the sliced liver tissues was fixed in 10% formalin solution for routine hematoxylin and eosin staining. Twenty milligrams of liver tissue powder prepared under liquid nitrogen were used for lipid analysis: lipids were extracted using isopropanol, and hepatic triglyceride content was measured using Wako L-type TG H assay (Wako Diagnostics, Richmond, VA), as previously described. The levels of hepatic inflammatory cytokines were measured using BD Cytometric Bead Array (catalog no. 552364, Mouse Inflammation Kit), according to the manufacturer's protocol. Caspase-3 activity was measured using EnzChek Caspase-3 assay kit (Invitrogen, catalog no. E13184). Frozen liver tissues were homogenized with the lysis buffer, and the lysates were then incubated in the reaction buffer, according to the manufacturer's protocol. Fluorescence was measured at an excitation wavelength of 496 nm and emission wavelength of 520 nm.
Measurement of hepatic PP2A activity.
The measurement of hepatic PP2A activity was performed by using the PP2A immunoprecipitation phosphatase assay kit (Millipore, CA), as previously described. Threonine phosphopeptide (K-R-Pt-I-R-R) was used as the PP2A substrate. In brief, the cells were harvested in lysis buffer (0.5 M Tris·HCl, pH 7.4, 1.5 M NaCl, 2.5% deoxycholic acid, 10% Nonidet P-40, 10 mM EDTA), 1 mM PMSF, and protease inhibitors. Supernatants were incubated with anti-PP2A (C subunit, clone 1D6]) and protein A agarose at 4°C for 2 h with constant rocking. The immunoprecipitates were then washed three times with Tris-buffered saline and diluted phosphopeptide (final concentration 750 μM), as well as Ser/Thr assay buffer was added. The mixtures were incubated for 10 min at 30°C in a shaking incubator and then briefly centrifuged, and 25 μl of the samples were transferred to 96-well microtiter plate. PP2A activities were determined by the addition of the Malachite Green Phosphate Detection Solution into the mixtures and measuring the absorbance at 650 nm. The absorbance values of each sample were compared with negative controls containing no PP2A enzyme activity.
Total RNA isolation and quantitative RT-PCR.
Total RNA was prepared from liver tissue using an Absolutely RNA RT-PCR Miniprep kit (Stratagene, Cedar Creek, TX). Reverse transcription of 1 μg total RNA to cDNA was performed using the StrataScript qPCR cDNA synthesis kit (Stratagene). Real-time quantitative polymerase chain reaction amplification was performed in a Stratagene MX 3005P thermal cycler (La Jolla, CA) using RT2 SYBR Green qPCR Master Mix. Primers for SYBR Green-based real-time PCR were purchased from SuperArray Bioscience (Frederick, MD). The relative amount of target mRNA was calculated using the comparative cycle threshold method and normalizing each target gene with cycle threshold of housekeeping gene, GAPDH.
Western blotting.
Sixty milligrams of whole liver tissue powder prepared under liquid nitrogen were homogenized with radioimmunoprecipitation assay buffer. Protein concentrations were determined by the Bio-Rad assay. Equal amounts of protein (normally 20 μg of protein, unless otherwise stated) were separated on SDS-polyacrylamide gels, transferred to a nitrocellulose membrane by the wet blotting method, and probed with antibodies as indicated. The amounts of bound antibodies were accessed by the peroxidase activity of horseradish peroxidase-conjugated secondary antibody, as detected by chemiluminescence with Lumi-light Western blotting substrate (Amersham Biosciences, Piscataway, NJ). The intensity of the individual bands on Western blots were measured by PhosphoImager and analyzed with ImageQuant (Amersham Biosciences) software analysis.
Luminex assay.
Bead-based multiplex ELISAs were used to examine the Akt signaling pathway in the hepatic tissue (catalog nos. LHO0001 and LHO0002, Akt Pathway Phospho 7-plex panel and total 7-plex panel; Invitrogen, Camarillo, CA). The following phosphorylated proteins, as well as the total levels of each protein, were measured according to the manufacturer's protocol: insulin receptor (IR), IGF-I receptor (IGF-IR), insulin receptor substrate-1 (IRS-1), Akt, glycogen synthase kinase-3β (GSK-3β), p70S6 kinase (p70S6K), and proline-rich Akt substrate 40 (PRAS40), and pYpY1162/1163-IR, pYpY1135/1136-IGF-IR, pS312-IRS-1, pS473-Akt, pS9-GSK-3β, pTpS421/424-p70S6K, and pT246-PRAS40. Samples containing 200 μg protein were incubated with the beads, and captured antigens were detected with biotinylated secondary antibody and phycoerythrin-conjugated streptavidin. Plates were read in a Luminex 100 system. Data are expressed as fluorescence light units in reference to respective protein standards corrected for total hepatic protein concentration.
Glucose tolerance test.
At the 27th day of feeding, glucose tolerance tests were performed in one-half of the mice in each group. Mice had been fasted for 6 h. Glucose (1 g/kg body wt) was given by intraperitoneal injection. Tail blood glucose was measured at 0, 15, 30, 60, and 120 min with a glucometer (Accu-Chek; Roche, Indianapolis, IN).
Data analysis.
All data are presented as means ± SE. Statistical significance was calculated with the Student t-test or ANOVA analysis, followed by post hoc testing with least squares difference, when appropriate. P < 0.05 was considered statistically significant.
RESULTS
Effects of imipramine on hepatic steatosis and glucose tolerance in ethanol-fed mice.
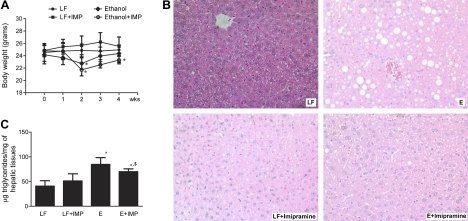
The alcohol-fed mice were statistically lighter after 2 wk on the diet, but they regained the weight steadily after that time point, and there was no effect of imipramine administration on this weight gain. Imipramine had no effect on the weight of the control-fed animals. The alcohol-fed animals treated with or without imipramine weighed the same at the time of death, although the alcohol + imipramine animals were lighter than the control group (Fig. 1A). Histological analysis of the livers (Fig. 1B) revealed prominent lipid accumulation in the livers of ethanol-fed animals compared with controls. As previously shown in our and other studies with mice fed the Lieber-Decarli diet, we did not observe evidence of inflammation on hepatic histology. Liver sections of pair-fed mice given imipramine intraperitoneally were similar to those of controls. Ethanol-fed mice had the expected steatosis; however, cotreatment with imipramine for the last 2 wk resulted in improvement in hepatic steatosis. Hepatic triglycerides were significantly elevated by about twofold by ethanol feeding; ethanol-fed animals receiving imipramine had a significant reduction in hepatic triglyceride, which remained above that in the control group (Fig. 1C).
Fig. 1.
Comparative body weight (A), liver histology (B), and hepatic triglyceride (C) of control, ethanol (E) fed, imipramine (IMP)-treated, and E + IMP-treated mice. A: while the alcohol-fed mice lost weight between weeks 1 and 2, there was no difference in the mean body weight in E-fed mice, with or without IMP treatment at the end of experiment. B: histological analysis of the livers revealed prominent lipid accumulation without significant inflammation in the livers of E-fed animals compared with controls. However, cotreatment with IMP for the last 2 wk resulted in improvement in hepatic steatosis. C: hepatic triglycerides were significantly elevated by about twofold by E feeding; E-fed animals receiving IMP had a significant reduction in hepatic triglyceride, which remained above that in the control group. LF, liquid fed. Values are means ± SE. *Significant difference vs. control, P < 0.05. $ P < 0.05 compared with E-treated group.
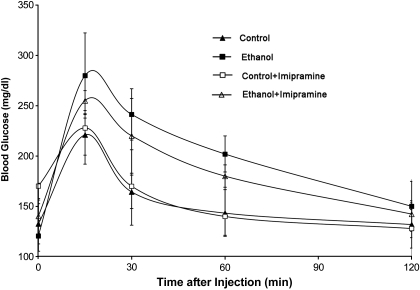
A random subset of the ethanol-fed animals was tested for glucose tolerance after 27 days of ethanol feeding (Fig. 2). There was no difference in the area under the curve between the control (307 ± 45 min·mg·dl−1) and imipramine treatment groups (311 ± 68 min·mg·dl−1). Ethanol feeding for 4 wk induced impaired glucose tolerance compared with controls (402 ± 40 min·mg·dl−1), and this was modestly improved with imipramine treatment (370 ± 31 min·mg·dl−1; n = 4 mice/group; P < 0.05) (Fig. 2).
Fig. 2.
Effect of E and IMP on glucose tolerance. Glucose tolerance test of mice fed control or E liquid diets, with and without IMP treatment, is shown. At the 27th day of feeding, after 6-h fasting, glucose (1 g/kg body wt) was administrated by intraperitoneal injection. Blood glucose levels were measured at the indicated times. Values are means ± SE, with n = 4 mice per group. There was no difference in the area under the curve between the control (307 ± 45 min·mg·dl−1) and IMP treatment groups (311 ± 68 min·mg·dl−1). E feeding induced impaired glucose tolerance compared with controls, and this was modestly improved with IMP treatment (402 ± 40 vs. 370 ± 31 min·mg·dl−1; n = 4 mice/group; P < 0.05).
Effect of ethanol and imipramine treatment on hepatic ceramide concentration and ASMase and PP2A activity.
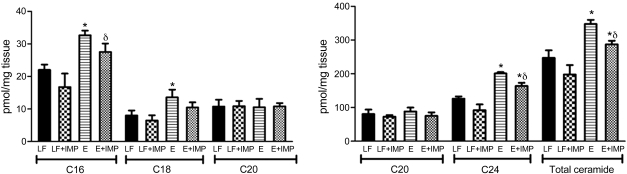
We previously showed total hepatic ceramide concentration was increased in ethanol-fed mice, with the largest increases observed for C16 and C18 ceramide species (16). In this report, in addition to C16 (32 ± 2 vs. 22 ± 2 pmol/mg tissue) and C18 ceramide (14 ± 2 vs. 8 ± 2 pmol/mg tissue), we also found that the levels of C24 ceramide were significantly higher in the ethanol group compared with controls (201 ± 4 vs. 126 ± 7 pmol/mg tissue, P < 0.05). Imipramine treatment significantly decreased total hepatic ceramide concentration compared with that of controls (198 ± 28 vs. 247 ± 22 pmol/mg tissue). Lipidomic profiling of individual ceramide species indicated that, whereas there was a significant decrease in total ceramide concentrations in response to inhibition of ceramide synthesis in ethanol-fed mice treated with and without imipramine (287 ± 11 vs. 348 ± 12 pmol/mg tissue or a 17% decrease), the magnitude and specificity of inhibition on each ceramide species differed. A significant decrease was observed for C16 (28 ± 3 vs. 33 ± 2 pmol/mg tissue, or 16% decrease) and C24 (164 ± 9 vs. 201 ± 4 pmol/mg tissue, or 18% decrease). The levels of hepatic ceramide profiles in response to imipramine treatment are shown in Fig. 3.
Fig. 3.
Effect of E and IMP treatment on hepatic ceramide contents. At the time of death, liver tissues were harvested and immediately freeze-clamped with Wollenberger tongs at the temperature of liquid nitrogen, powdered under liquid nitrogen with a mortar and pestle, and stored at −80°C. Ten milligrams of liver tissue powder prepared under liquid nitrogen were used for the measurement of ceramide contents with mass spectrometry. In addition to C16 and C18 ceramide, the levels of C24 ceramide were significantly higher in E group compared with controls. IMP treatment significantly decreased total hepatic ceramide concentration compared with controls (198 ± 28 vs. 247 ± 22 pmol/mg tissue). Lipidomic profiling of individual ceramide species indicated that, whereas there was a significant decrease in total ceramide concentrations in response to inhibition of ceramide synthesis in E-fed mice treated with and without IMP, the magnitude and specificity of inhibition on each ceramide species differed. A significant decrease was observed for C16 and C24. Values are means ± SE. *Significant difference vs. control, P < 0.05. δP < 0.05 compared with E-treated group.
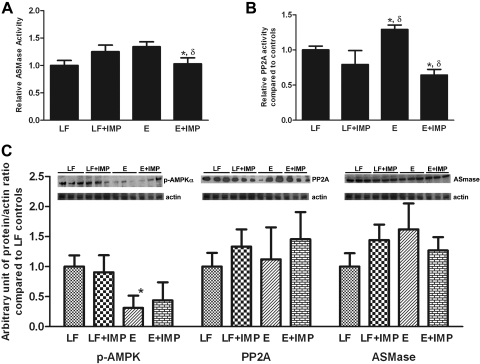
In agreement with previous in vitro and in vivo data, hepatic ASMase activity in ethanol-fed mice was significantly higher than in controls (controls: 1.0 ± 0.1 vs. ethanol: 1.3 ± 0.1, P < 0.05). As we had predicted, the activity of ASMase was returned to normal by treatment with imipramine (ethanol-fed: 1.3 ± 0.1 vs. ethanol-fed with imipramine 1.0 ± 0.1, P < 0.05) (Fig. 4A). A well-known stimulus for ceramide generation is activation of the TNF-α signaling pathway (35), and increased hepatic TNF-α is observed to accompany the hepatic injury that is produced by intragastric feeding of ethanol in the Tsukamoto-French model (37). TNF-α levels are variably increased in animals fed lower amounts of ethanol (4). We, therefore, measured TNF-α levels in the liver samples. Ethanol did not significantly increase TNF-α levels compared with controls (control, 5.3 ± 1.7 pg/mg protein vs. ethanol, 10.3 ± 3.3 pg/mg protein). Similarly, imipramine injection had no effect on hepatic TNF-α levels (imipramine group, 4.7 ± 2.9 pg/mg protein vs. ethanol plus imipramine, 6.7 ± 5.7 pg/mg protein). Thus, in this experimental model, TNF-α signaling probably did not contribute to the ethanol-induced increase in ceramide generation.
Fig. 4.
Effect of E and IMP treatment on acidic sphingomyelinase (ASMase), protein phosphatase 2A (PP2A), and AMPK. A: in agreement with previous in vitro and in vivo data, hepatic ASMase activity in E-fed mice was significantly higher than in controls and was normalized by treatment with IMP. B: hepatic PP2A activity in E-fed mice was significantly higher than that of controls, and the activity in E-fed animals was lowered by concomitant IMP treatment. IMP alone appeared to lower PP2A activity below the control level, but this did not achieve statistical significance. C: the level of phospho-AMPK (pAMPK) was markedly reduced by E feeding, but IMP did not reverse this effect. E and IMP treatment had no effect on the protein levels of PP2A or ASMase. *Significant difference vs. control, P < 0.05. δP < 0.05 compared with E-treated group.
Since ceramide activates PP2A, we next determined the effects of ethanol on hepatic PP2A activity. Hepatic PP2A activity in ethanol-fed mice was significantly higher than that of controls (1.3 ± 0.1 vs. 1.0 ± 0.05, P < 0.05), and the activity in ethanol-fed animals was lowered by concomitant imipramine treatment (ethanol: 1.3 ± 0.1 vs. ethanol with imipramine: 0.6 ± 0.1, P < 0.05). Imipramine alone appeared to lower PP2A activity below the control level, but this did not achieve statistical significance (Fig. 4B). The level of phospho-AMPK was determined in freeze-clamped liver samples from the four groups of animals. As expected, phospho-AMPK was markedly reduced by ethanol feeding, but we were surprised that imipramine did not reverse this effect. Ethanol and imipramine treatment had no effect on the protein levels of PP2A or ASMase (Fig. 4C).
Effects of ethanol and imipramine treatment on insulin signaling and activation of stress kinases.
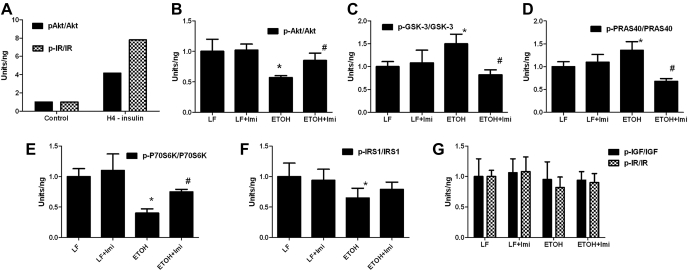
Inhibitory effects of ethanol on insulin signaling are variously reported to result from reduced binding of insulin to its receptor (6, 7), reduced tyrosine phosphorylation (which corresponds to activation of IR tyrosine kinase) (20), and inhibition of signaling through IRS-1 (26), phosphatidylinositol 3-kinase-Akt (9, 24), PRAS40-mammalian target of rapamycin-S6 kinase (11), and ERK MAPK (36). Administration of a ceramide analog to rats reproduced many of the effects of ethanol feeding on hepatic insulin signaling (5), specifically, ceramide treatment reduced the relative levels of IRS-1 and Akt phosphorylation and increased levels of pS9-GSK-3β and pS9-GSK-3β/GSK-3β compared with liver samples from rats fed an inactive dihydroceramide analog. We, therefore, analyzed the effect of ethanol feeding and imipramine treatment on insulin signaling using the Luminex bead-based multiplex ELISA assay (Fig. 5). Ethanol feeding did not affect the levels of phospho-IR or phospho-IGF-IR, but did reduce the levels of phospho-IRS1Ser312. Ethanol feeding also reduced p-Akt Ser473 levels and increased the level of pS9-GSK-3β. These changes in phosphorylation mimicked those seen with ceramide administration (above) and were reversed by imipramine, suggesting that ceramide mediated this effect of ethanol. There were two additional significant effects of ethanol feeding that were reversed by imipramine: decreased phosphorylation of pTpS421/424-p70S6K and increased phosphorylation of pT246-PRAS40.
Fig. 5.
Effects of E and IMP (Imi) treatment on insulin signaling. The effect of E and IMP treatment on insulin signaling was determined using Luminex bead-based multiplex ELISA assay (see text for detail). E feeding reduced phospho-Akt (p-Akt) Ser473 levels (B) and increased the level of glycogen synthase kinase-3β (GSK-3β) Ser9 phosphorylation (C). These changes were reversed by IMP. There were two additional significant effects of E feeding that were reversed by IMP: decreased phosphorylation of pTpS421/424-p70S6K (p70S6 kinase; E) and increased phosphorylation of pT246-PRAS40 (proline-rich Akt substrate 40; D). E feeding did not affect the levels of phospho-IR (insulin receptor) or phospho-IGF-I receptor (G), but did reduce the levels of phospho-IR substrate-1 (p-IRS-1) Ser312 (F). A: hepatoma cells treated with insulin were used as the positive control of the assay. Values are means ± SE. *Significant difference vs. control, P < 0.05. #P < 0.05 compared with E-treated group.
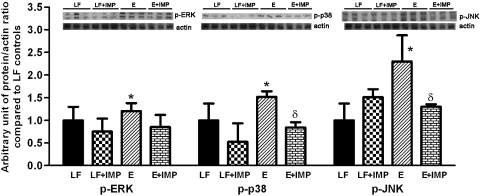
Ceramide induces apoptosis in many cell models, and one pathway is via activation of apoptosis signal-regulating kinase-1 (Ask1) (21). This enzyme is responsive to redox stress through reductions in thioredoxin and is also inhibited by phosphorylation by Akt. It may be a master regulator of stress responses via its ability to activate p38 and JNK via activation of MKK3/6 and SEK1/MKK4; it does not activate Erk (1). Therefore, we determined the effect of ethanol feeding and imipramine treatment on these stress kinases (Fig. 6). Ethanol feeding increased the levels of the phosphorylated forms of ERK slightly and increased phospho-p38 and phospho-JNK substantially more. The levels of phospho-p38 and phospho-JNK were reduced by treatment with imipramine. These results are consistent with ethanol activating Ask1 by way of ceramide generation and/or inhibition of Akt. Commercially available antibodies detected Ask1 in the liver samples, but we could not detect phospho-Ask1 either in the liver samples or in extracts of hepatoma cells using phospho-specific antibodies (from Santa Cruz Biotechnology, catalog no. sc-166967 and Abcam, catalog no. ab47304). Given the increased activity of the stress kinases and reduced activity of Akt, we expected to see activation of apoptosis; however, the caspase-3 activity was not increased in the ethanol-fed animals (not shown).
Fig. 6.
Effects of E and IMP treatment on stress kinases. The levels of ERK, p38, JNK and their phosphorylated forms (p-ERK, p-p38, p-JNK) were determined by Western blotting. E feeding increased the levels of the phosphorylated forms of ERK slightly, and increased p-p38 and p-JNK substantially. The levels of p-p38 and p-JNK were reduced by treatment with IMP. Values are means ± SE. *Significant difference vs. control, P < 0.05. δP < 0.05 compared with E-treated group.
DISCUSSION
Chronic ethanol feeding has a multitude of effects on the liver that vary with the level and duration of consumption. These include inhibition of fatty acid oxidation, increased de novo lipid synthesis, impaired export of triglyceride in the form of VLDL, insulin resistance, mitochondrial dysfunction, endoplasmic reticulum and oxidative stress, apoptosis, and ultimately inflammation and fibrosis (29). It is difficult to account for all of these effects by a single mechanism; however, in vitro studies have shown that ethanol increases hepatic levels of ceramide, and that this was associated with increased activity of PP2A (16). Ceramide and activated protein phosphatases could affect multiple signaling and metabolic pathways. Ceramide administration to rats for approximately 1 mo reproduced many features of insulin resistance associated with ethanol administration (5, 30). Others have shown that use of the ASMase inhibitor amitriptyline limited formation of ceramide in the liver of LEC rats (a genetic model for Wilson disease), reduced histological damage to the liver (steatosis, fibrosis, and nuclear pyknosis), and increased their survival (13). Hence, ceramide generation might be a common mechanism underlying several of the effects of ethanol on the liver. To better understand if ceramide and PP2A activation account for the pleiotropic effects of ethanol on the liver, we examined the ability of the ASMase inhibitor, imipramine, to block ceramide generation and abrogate the effects of ethanol on lipid and carbohydrate metabolism. As a proof of concept, we had previously demonstrated that imipramine reduced the level of ceramide in cultured hepatoma cells incubated with ethanol (16).
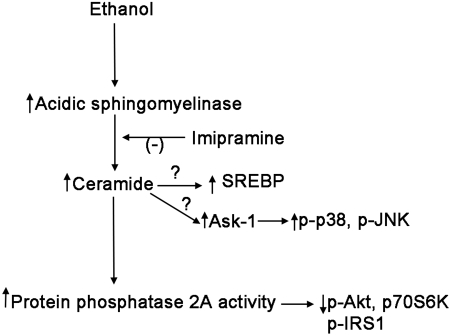
Ethanol feeding led to the development of fatty liver and glucose intolerance (Figs. 1 and 2). We found that 2 wk of daily imipramine injection reduced ASMase activity, reduced ceramide levels, and concomitantly reduced PP2A activity that were elevated by an ethanol-containing diet (Fig. 3). This was associated with reduced triglyceride accumulation and modest improvement in glucose tolerance. These results bolster the hypothesis that ethanol-induced activation of ASMase contributes to the induction of fatty liver and reveals a target for therapeutic intervention. The increase in ASMase activity was not due to increased levels of TNF-α in the liver. Alternative pathways that might be responsible for ASMase activation include oxidative and endoplasmic reticulum stress: studies on the mechanism by which ethanol activates ASMase are underway. We were surprised that the reduced level of active phospho-AMPK was not restored by treatment of the ethanol-fed animals with imipramine (Fig. 3). This suggests that ethanol blocks AMPK activation by mechanisms other than solely activation of PP2A, such as by inhibition of the activation pathway leading to LKB1 activation (17), and demonstrating that steatosis can be improved despite low activity of AMPK. We speculate that the reduction in steatosis seen in imipramine-treated mice was due to reduced activity of sterol regulatory element binding protein (SREBP)-1c (see Fig. 7), based on the observation that TNF-α-activation of NSMase or treatment of liver cells with SMase or ceramide increased the proteolytic maturation of SREBP-1c (14). The mechanism for this effect is unknown: caspase-3 has been reported to cleave and activate SREBP-1 (23) and to be activated by ceramide (19); however, caspase-3 activity was not increased by ethanol feeding.
Fig. 7.
Scheme for potential mechanisms underlying the protective action of IMP against alcoholic fatty liver. Our study suggests that the activation of ASMase and generation of ceramide in response to E feeding may underlie several effects of E, such as development of steatosis, glucose intolerance, impaired insulin signaling, and activation of stress kinases. Treatment with IMP inhibited the activity of ASMase, reduced hepatic ceramide content, and inhibited the activity of PP2A. IMP reversed several effects of E on the Akt and insulin signaling pathway, presumably from its effect on PP2A. The levels of p-p38 and p-JNK, but not p-ERK, were reduced by treatment with IMP. Further studies are needed to further determine the mechanism of IMP on these stress kinases and on the possibility that ceramide activates sterol regulatory element binding protein (SREBP)-1 in the liver of alcohol-fed mice. Ask1, apoptosis signal-regulating kinase-1.
We also examined the effects of ethanol and of imipramine treatment on several additional pathways potentially affected by ceramide or PP2A activation. We found that the effect of ethanol feeding was similar to the effect of ceramide administration on phosphorylation of IRS-1, Akt, and GSK-3β, and these effects were reversed by imipramine, suggesting that these effects of ethanol are mediated by ceramide, and that they may be related to the ethanol-induced glucose intolerance. The mechanisms underlying these changes in insulin signaling are uncertain. Phosphorylation of IRS-1Ser312 is associated with impaired insulin signaling, so lower levels of phosphorylation induced by ethanol would not be expected to contribute to the observed impaired glucose tolerance. Treatment of adipocytes with okadaic acid resulted in hyperphosphorylation of IRS-1 at serine/threonine residues (3). This suggests that reduced phosphorylation of IRS-1 in ethanol-treated liver may be a result of activation of PP2A.
Current mechanisms for ethanol-induced Akt inhibition include induction of PTEN (phosphatase and tensin homolog deleted on chromosome 10) (27); however, ceramide can reduce the phosphorylation of Akt independent of PTEN, as demonstrated in PTEN-deficient cell lines (39), suggesting again that activation of PP2A can lead to dephosphorylation of phospho-Akt (39). Whether PTEN induction is also mediated by ceramide or other factors requires additional study. Increased phosphorylation of pS9-GSK-3β is of interest. Low-dose ethanol feeding of rats was shown to increase GSK-3β phosphorylation, while high-dose intragastric administration of ethanol reduced phospho-GSK-3β (9). Phosphorylation of this site inhibits its activity [hence activation of GSK-3β by high levels of ethanol intake might explain the observed hepatic glycogen depletion (31)]. Ethanol feeding affected two other participants in the insulin signaling cascade in an imipramine-sensitive fashion: decreased phosphorylation of p70S6K and increased phosphorylation of PRAS40Thr246. The phosphorylation sites for p70S6K detected with this assay (pThrpSer 421/424) correlate with full activation of the kinase, but are not the targets of mammalian target of rapamycin C1 (which is activated by Akt); the kinases responsible for phosphorylation of Thr/Ser 421/424 are not known. Once again, it is attractive to speculate that activation of PP2A is responsible for this effect of ethanol. Consistent with this hypothesis, low concentrations of okadaic acid (50 nM) markedly increased phosphorylation of p70S6K, although the specific phosphorylated sites were not identified (15). The increased phosphorylation of pT246-PRAS40 (another target of Akt) suggested that ethanol activated non-Akt pathways capable of phosphorylating PRAS40 or somehow inhibited its dephosphorylation.
Our study also showed the activation of several stress kinases in ethanol-fed mice. Phosphorylated (active) forms of p38 and JNK were increased by ethanol feeding to a greater extent than ERK, and this was reversed by imipramine treatment. Additional studies will be needed to determine how imipramine ameliorates the effect of ethanol on these kinases; one attractive possibility is through interactions at the level of the Ask1 (see Fig. 7) (32).
Since increased activity of the stress kinases and decreased activity of Akt would be expected to predispose the cells to apoptosis, we examined activation of caspase-3. This effector caspase was not activated, which suggests that either the degree of activation of proapoptotic pathways did not reach a threshold to induce cell death, or that apoptotic cells were cleared efficiently from the liver. In either event, it is possible that increased ceramide generated in response to the ethanol diet makes the hepatocytes more sensitive to additional insults [such as LPS-induced TNF-α (30) or more severe oxidative stress] that appear to be important in the transition from simple fatty liver to steatohepatitis.
In summary, this study suggests that the activation of ASMase and generation of ceramide in response to ethanol feeding may underlie several effects of ethanol, namely, development of steatosis, glucose intolerance, impaired insulin signaling, and activation of stress kinases (Fig. 7). Hepatic steatosis, once considered benign, is now being recognized as a condition that may lead to steatohepatitis, fibrosis, and ultimately cirrhosis. Since small-molecule inhibitors of ASMase are available for use in humans, this may provide a therapeutic target to treat alcoholic steatosis and thus reduce the hepatic morbidity of heavy ethanol consumption.
GRANTS
This study is supported by Veterans Administration Young Investigator Award/Indiana Institute for Medical Research, K08 AA016570 from the National Institutes of Health/National Institute on Alcohol Abuse and Alcoholism (NIAAA), Central Society for Clinical Research Career development award, and Research Support Fund Grant (S. Liangpunsakul) and R01 AA15070, P60 AA07611 from the NIAAA (D. W. Crabb).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.L. conception and design of research; S.L., Y.R., R.A.R., and Z.Z. performed experiments; S.L. and Y.R. analyzed data; S.L. and Y.X. interpreted results of experiments; S.L. prepared figures; S.L. and D.W.C. drafted manuscript; S.L. and D.W.C. edited and revised manuscript; S.L., Y.X., and D.W.C. approved final version of manuscript.
REFERENCES
- 1. Arboleda G, Cardenas Y, Rodriguez Y, Morales LC, Matheus L, Arboleda H. Differential regulation of AKT, MAPK and GSK3beta during C(2)-ceramide-induced neuronal death. Neurotoxicology 31: 687–693, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Chavant F, Deguil J, Pain S, Ingrand I, Milin S, Fauconneau B, Perault-Pochat MC, Lafay-Chebassier C. Imipramine, in part through tumor necrosis factor alpha inhibition, prevents cognitive decline and beta-amyloid accumulation in a mouse model of Alzheimer's disease. J Pharmacol Exp Ther 332: 505–514, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Clark SF, Molero JC, James DE. Release of insulin receptor substrate proteins from an intracellular complex coincides with the development of insulin resistance. J Biol Chem 275: 3819–3826, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Cohen JI, Roychowdhury S, DiBello PM, Jacobsen DW, Nagy LE. Exogenous thioredoxin prevents ethanol-induced oxidative damage and apoptosis in mouse liver. Hepatology 49: 1709–1717, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de la Monte SM, Tong M, Nguyen V, Setshedi M, Longato L, Wands JR. Ceramide-mediated insulin resistance and impairment of cognitive-motor functions. J Alzheimers Dis 21: 967–984, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de la Monte SM, Yeon JE, Tong M, Longato L, Chaudhry R, Pang MY, Duan K, Wands JR. Insulin resistance in experimental alcohol-induced liver disease. J Gastroenterol Hepatol 23: e477–e486, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Denucci SM, Tong M, Longato L, Lawton M, Setshedi M, Carlson RI, Wands JR, de la Monte SM. Rat strain differences in susceptibility to alcohol-induced chronic liver injury and hepatic insulin resistance. Gastroenterol Res Pract pii: 312790, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fernandez-Checa JC, Colell A, Mari M, Garcia-Ruiz C. Ceramide, tumor necrosis factor and alcohol-induced liver disease. Alcohol Clin Exp Res 29: 151S–157S, 2005 [PubMed] [Google Scholar]
- 9. He J, de la Monte S, Wands JR. Acute ethanol exposure inhibits insulin signaling in the liver. Hepatology 46: 1791–1800, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Holland WL, Summers SA. Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocr Rev 29: 381–402, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hong-Brown LQ, Brown CR, Kazi AA, Huber DS, Pruznak AM, Lang CH. Alcohol and PRAS40 knockdown decrease mTOR activity and protein synthesis via AMPK signaling and changes in mTORC1 interaction. J Cell Biochem 109: 1172–1184, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J 353: 417–439, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lang PA, Schenck M, Nicolay JP, Becker JU, Kempe DS, Lupescu A, Koka S, Eisele K, Klarl BA, Rubben H, Schmid KW, Mann K, Hildenbrand S, Hefter H, Huber SM, Wieder T, Erhardt A, Haussinger D, Gulbins E, Lang F. Liver cell death and anemia in Wilson disease involve acid sphingomyelinase and ceramide. Nat Med 13: 164–170, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Lawler JF, Jr, Yin M, Diehl AM, Roberts E, Chatterjee S. Tumor necrosis factor-alpha stimulates the maturation of sterol regulatory element binding protein-1 in human hepatocytes through the action of neutral sphingomyelinase. J Biol Chem 273: 5053–5059, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Leicht M, Simm A, Bertsch G, Hoppe J. Okadaic acid induces cellular hypertrophy in AKR-2B fibroblasts: involvement of the p70S6 kinase in the onset of protein and rRNA synthesis. Cell Growth Differ 7: 1199–1209, 1996 [PubMed] [Google Scholar]
- 16. Liangpunsakul S, Sozio MS, Shin E, Zhao Z, Xu Y, Ross RA, Zeng Y, Crabb DW. Inhibitory effect of ethanol on AMPK phosphorylation is mediated in part through elevated ceramide levels. Am J Physiol Gastrointest Liver Physiol 298: G1004–G1012, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liangpunsakul S, Wou SE, Zeng Y, Ross RA, Jayaram HN, Crabb DW. Effect of ethanol on hydrogen peroxide-induced AMPK phosphorylation. Am J Physiol Gastrointest Liver Physiol 295: G1173–G1181, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mathias S, Pena LA, Kolesnick RN. Signal transduction of stress via ceramide. Biochem J 335: 465–480, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mizushima N, Koike R, Kohsaka H, Kushi Y, Handa S, Yagita H, Miyasaka N. Ceramide induces apoptosis via CPP32 activation. FEBS Lett 395: 267–271, 1996 [DOI] [PubMed] [Google Scholar]
- 20. Mohr L, Tanaka S, Wands JR. Ethanol inhibits hepatocyte proliferation in insulin receptor substrate 1 transgenic mice. Gastroenterology 115: 1558–1565, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Mullen TD, Obeid LM. Ceramide and apoptosis: exploring the enigmatic connections between sphingolipid metabolism and programmed cell death. Anticancer Agents Med Chem. In press. [DOI] [PubMed] [Google Scholar]
- 22. Naudon L, Hotte M, Jay TM. Effects of acute and chronic antidepressant treatments on memory performance: a comparison between paroxetine and imipramine. Psychopharmacology (Berl) 191: 353–364, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Pai JT, Brown MS, Goldstein JL. Purification and cDNA cloning of a second apoptosis-related cysteine protease that cleaves and activates sterol regulatory element binding proteins. Proc Natl Acad Sci U S A 93: 5437–5442, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pang M, de la Monte SM, Longato L, Tong M, He J, Chaudhry R, Duan K, Ouh J, Wands JR. PPARdelta agonist attenuates alcohol-induced hepatic insulin resistance and improves liver injury and repair. J Hepatol 50: 1192–1201, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rinella ME, Elias MS, Smolak RR, Fu T, Borensztajn J, Green RM. Mechanisms of hepatic steatosis in mice fed a lipogenic methionine choline-deficient diet. J Lipid Res 49: 1068–1076, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sasaki Y, Wands JR. Ethanol impairs insulin receptor substrate-1 mediated signal transduction during rat liver regeneration. Biochem Biophys Res Commun 199: 403–409, 1994 [DOI] [PubMed] [Google Scholar]
- 27. Shulga N, Hoek JB, Pastorino JG. Elevated PTEN levels account for the increased sensitivity of ethanol-exposed cells to tumor necrosis factor-induced cytotoxicity. J Biol Chem 280: 9416–9424, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Song L, Che W, Min-Wei W, Murakami Y, Matsumoto K. Impairment of the spatial learning and memory induced by learned helplessness and chronic mild stress. Pharmacol Biochem Behav 83: 186–193, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Sozio MS, Liangpunsakul S, Crabb D. The role of lipid metabolism in the pathogenesis of alcoholic and nonalcoholic hepatic steatosis. Semin Liver Dis 30: 378–390, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Teruel T, Hernandez R, Lorenzo M. Ceramide mediates insulin resistance by tumor necrosis factor-alpha in brown adipocytes by maintaining Akt in an inactive dephosphorylated state. Diabetes 50: 2563–2571, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Van Horn CG, Ivester P, Cunningham CC. Chronic ethanol consumption and liver glycogen synthesis. Arch Biochem Biophys 392: 145–152, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Wu D, Cederbaum A. Activation of ASK-1 and downstream MAP kinases in cytochrome P4502E1 potentiated tumor necrosis factor alpha liver injury. Free Radic Biol Med 49: 348–360, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wu Y, Song P, Xu J, Zhang M, Zou MH. Activation of protein phosphatase 2A by palmitate inhibits AMP-activated protein kinase. J Biol Chem 282: 9777–9788, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Xu Y, Lin D, Li S, Li G, Shyamala SG, Barish PA, Vernon MM, Pan J, Ogle WO. Curcumin reverses impaired cognition and neuronal plasticity induced by chronic stress. Neuropharmacology 57: 463–471, 2009 [DOI] [PubMed] [Google Scholar]
- 35. Yang SQ, Lin HZ, Yin M, Albrecht JH, Diehl AM. Effects of chronic ethanol consumption on cytokine regulation of liver regeneration. Am J Physiol Gastrointest Liver Physiol 275: G696–G704, 1998 [DOI] [PubMed] [Google Scholar]
- 36. Yeon JE, Califano S, Xu J, Wands JR, de la Monte SM. Potential role of PTEN phosphatase in ethanol-impaired survival signaling in the liver. Hepatology 38: 703–714, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Yin M, Wheeler MD, Kono H, Bradford BU, Gallucci RM, Luster MI, Thurman RG. Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology 117: 942–952, 1999 [DOI] [PubMed] [Google Scholar]
- 38. You M, Matsumoto M, Pacold CM, Cho WK, Crabb DW. The role of AMP-activated protein kinase in the action of ethanol in the liver. Gastroenterology 127: 1798–1808, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Zinda MJ, Vlahos CJ, Lai MT. Ceramide induces the dephosphorylation and inhibition of constitutively activated Akt in PTEN negative U87mg cells. Biochem Biophys Res Commun 280: 1107–1115, 2001 [DOI] [PubMed] [Google Scholar]