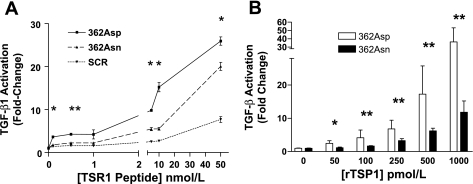
Fig. 4.
The 362Asn TSP1 mutation displays loss-of-function characteristics on latent TGF-β1 activation in the mink lung epithelial cell bioassay. A: latent TGF-β1 activation by synthetic 13-amino acid TSR1 peptides centered on the 362Asn TSP1 locus. At all concentrations wild-type TSR1 (362Asp) is a more potent activator compared with mutant TSR1 [362Asn, which activates more than scrambled peptide (SCR) only when ≥5 nM]. 362Asp effects were always greater than SCR effects (*P < 0.05). B: trimeric recombinant TSP1 (rTSP1) is a more potent activator of TGF-β1 (pM effects). The mutant isoform is weaker at all concentrations compared with the wild-type isoform (*P < 0.01, **P < 0.001). Activation is expressed as fold change compared with vehicle (zero). Symbols indicate statistical significance by ANOVA or unpaired t-tests. Results represent summed means ± SD of ≥4 experiments.