Figure 1. Msrs reduce S-stereoisomer but not R-stereoisomer forms of methylsulfinyl-containing drugs and natural compounds.
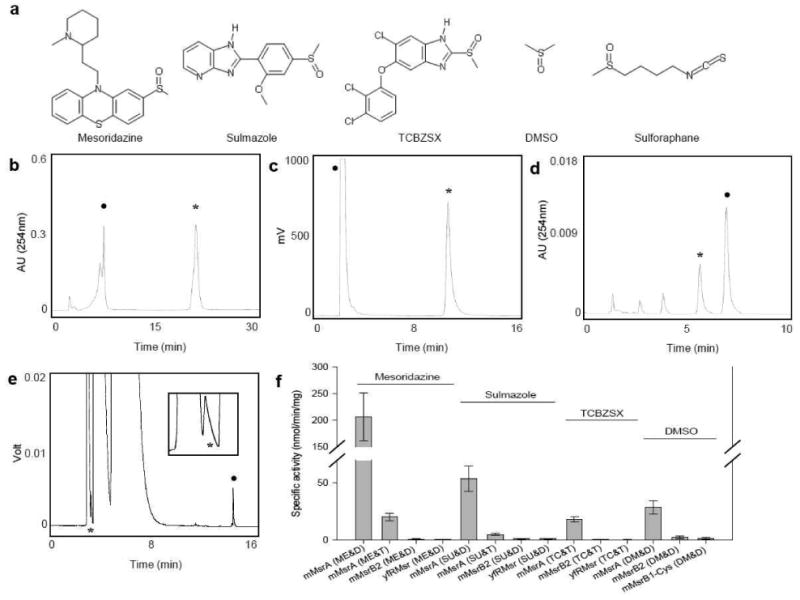
(A) Structures of representative methylsulfinyl-containing compounds. (B) HPLC analysis of mesoridazine reduction by mMsrA using DTT as a reductant. (C) HPLC analysis of sulmazole reduction by mMsrA with DTT as a reductant. Reduced and oxidized forms were measured by following fluorescence with the excitation at 330 nm and emission at 370 nm. (D) HPLC analysis of TCBZSX reduction by mMsrA with TrxR/Trx/NADPH. (E) GC analysis of DMSO reduction by mMsrA with DTT. The peak of dimethylsulfide, the reduced form of DMSO, is enlarged in the inset. In panels B-E, peaks of original substrates are marked with closed circles, and peaks of reduced forms with stars. (F) Specific activities of mMsrA, mMsrB2, and yfRMsr for reduction of mesoridazine, sulmazole, TCBZSX, and DMSO using either DTT or TrxR/Trx/NADPH as reductants. All measurements were repeated 3 times independently. Abbreviations used are: mesoridazine (ME), sulmazole (SU), TCBZSX (TC), DMSO (DM), DTT (D), TR/Trx/NADPH (T).
