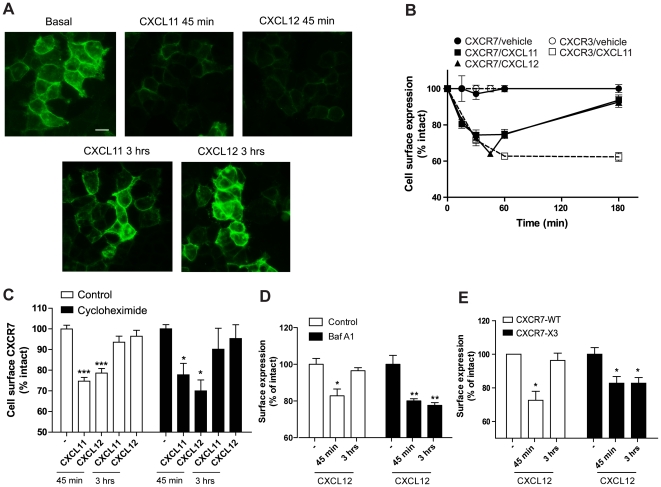
Figure 3. CXCR7 recycles to the cell surface after internalization.
(A) HEK293T stably expressing CXCR7 were stimulated with 10−8 M CXCL11, CXCL12 or vehicle for 45 min or 3 h and fixed immediately. CXCR7 was detected using the specific 11G8 antibody and an Alexa-488-conjugated secondary antibody. Scale bar represents 10 µm. (B) HEK293T cells expressing CXCR7 (filled symbols) or CXCR3 (open symbols) were incubated with CXCL11 (10−8 M, squares), CXCL12 (10−8 M, triangles) or vehicle (circles) for the indicated times. Cell surface receptor levels were detected by ELISA using CXCR7- or CXCR3-specific antibodies (11G8 and mAB160, respectively). Results were normalized to basal surface protein levels, and data represent the mean ± SEM of 4 experiments each performed in triplicate. (C) ELISA was performed as in B in cells pre-incubated for 2 h with the de novo protein synthesis inhibitor cycloheximide (10 µg/ml). (D) ELISA performed as in C on intact HEK293/CXCR7 cells treated with vehicle or 1 µM of bafilomycin A1 (Baf A1), 30 min prior to incubation with CXCL12. (E) C-terminal Ser/Thr clusters determine receptor fate after internalization. HEK293T cells were transiently transfected with CXCR7 wt (white bars) or with a chimeric receptor consisting of CXCR7 harboring the C-terminal sequence of CXCR3 (CXCR7-X3, filled bars). To assess the cell surface expression of the receptor, ELISA experiments were performed after 30 min or 3 hours of incubation with 10−8 M CXCL12. Data represent the mean ± SEM of 3 experiments each performed in triplicate. ***, p<0.001, **, p<0.01, and *, p<0.05 by one-way ANOVA and Bonferroni post test.