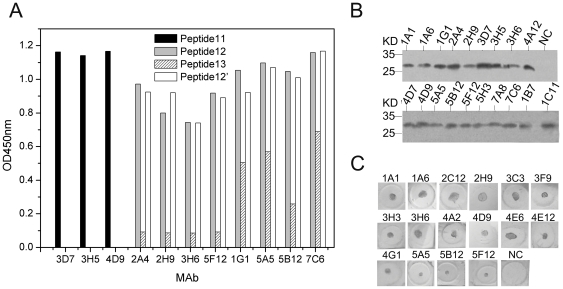
Figure 1. Reactivity of mAbs to 16mer peptides and native BP26 containing membrane protein extracts of B. melitensis M5-90.
(A) Bindings of mAbs to 16mer overlapping peptides in Peptide-ELISA. Twenty nine of 16mer overlapping peptides spanning the entire sequence of BP26 were coated to the plates with 5 µg/ml in CBS buffer (pH 9.6). The peptides bound plates were tested with 29 mAb supernatants of hybridomas. An HCV NS3 peptide coated wells were used as negative-controls. Cut off was defined above 2.1 folds of OD value to negative control. The dot line indicates the level of cut off. (B) Reactivity of mAbs to the SDS denatured native BP26 of NMP in Western-Blot. (C) Reactivity of mAbs to non-denatured native BP26 of NMP in Dot-ELISA. NC, a negative control of an un-related mAb to HCV rNS3.