Abstract
Ly108 (CD352) is a member of the Signaling Lymphocyte Activation Molecule (SLAM) family of receptors that signals through SLAM-associated protein (SAP), an SH2 domain protein that can function by the recruitment of Src family kinases or by competition with phosphatases. Ly108 is expressed on a variety of hematopoietic cells and with especially high levels on developing thymocytes. We find that Ly108 is constitutively tyrosine phosphorylated in murine thymi in a SAP- and Fyn kinase-dependent manner. Phosphorylation of Ly108 is rapidly lost after thymocyte disaggregation, suggesting dynamic contact-mediated regulation of Ly108. Similar to recent reports, we find at least 3 isoforms of Ly108 mRNA and protein in the thymus, which are differentially expressed in the thymi of C57Bl/6 and 129S6 mice that express the lupus-resistant and lupus-prone haplotypes of Ly108, respectively. Notably, the recently described novel isoform, Ly108-H1, is not expressed in mice having the lupus-prone haplotype of Ly108, but is expressed in C57Bl/6 mice. We further provide evidence for differential phosphorylation of these isoforms; the novel Ly108-H1 does not undergo tyrosine phosphorylation, suggesting it functions as a decoy isoform that contributes to the reduced overall phosphorylation of Ly108 seen in C57Bl/6 mice. Our studies suggest that Ly108 is dynamically regulated in the thymus and shed light on Ly108 isoform expression and phosphorylation.
Introduction
SLAM family members belong to the CD2 super family of transmembrane proteins that are highly expressed on hematopoietic cells and function in lymphocyte development, cytokine regulation, cytotoxicity, cell adhesion, and apoptosis. The SLAM family consists of six members, including the prototypic member SLAM/CD150 (SLAMF1), Ly9 (CD229, SLAMF3), 2B4 (CD244, SLAMF4), CD84 (SLAMF5), Ly108 (NTB-A (human), CD352, SLAMF6) and CRACC (CD319, SLAMF7), which signal through the SLAM associated protein (SAP). With the exception of 2B4, which interacts with CD48, all other SLAM family members are self-ligands (1-8) The SLAM family members are encoded in a gene cluster in syntenic regions of mouse and human chromosome one (9). Genetic studies of lupus-prone mouse strains, as well as genome-wide association studies in humans have implicated this region as an autoimmunity susceptibility locus (10-12). In particular, distinct Ly108-encoding haplotypes have been associated with the development of autoantibodies in lupus-prone strains of mice (13, 14). This difference was initially linked to the altered ratios of expression of two differentially spliced isoforms. Ly108-1 is preferentially represented in the SLAM haplotype of lupus -prone mice such as B6.Sle1b, which express the lupus-prone SLAM haplotype introgressed onto the C57Bl/6 background, as well as 129, A/J, BALB/cJ and NZW mice. Ly108-2 was found more highly represented in SLAM haplotype of lupus-resistant C57Bl/6 mice (13). An additional isoform, Ly108-3 has also been reported (15). Recently, a novel isoform of Ly108, Ly108-H1 has been described, which is preferentially expressed in lupus-resistant mice and can mitigate lupus progression when transferred to lupus-prone mice (16). However, the function and nature of signaling from this isoform has not been evaluated.
SLAM family members are known to signal by binding of the SH2 domain adaptor molecule SAP to intracellular immunoreceptor tyrosine-based switch motifs (ITSMs) in their cytoplasmic tails (17, 18). Studies on the prototypic member SLAM have shown that SAP functions by serving as an adaptor, simultaneously binding to an ITSM in SLAM by its SH2 domain and to the SH3 domain of the Src family kinase Fyn. Fyn recruitment by SAP leads to kinase activation and phosphorylation of tyrosines in the cytoplasmic tail of SLAM, which, in turn, serve as binding sites for other adaptors and proteins involved in downstream signaling (19-21). However, some data indicate that SAP may also function by competing with phosphatases such as SHP-1/2 and SHIP, which can propagate negative signaling from these receptors (17, 18, 22-26). It can thus be imagined that signaling from these receptors can become stimulatory or inhibitory depending on the expression of SAP.
The importance of SAP and its associated SLAM receptors is highlighted by the human primary genetic disorder X-Linked Lymphoproliferative disorder 1 (XLP1), in which mutations in the gene Sh2d1a (encoding SAP) disrupt SAP expression (17, 27, 28). XLP1 is a complex disorder characterized by dysregulated immune responses that are often triggered or exacerbated by infection with Epstein-Barr virus, resulting in fulminant infectious mononucleosis, lymphomas and dysgammaglobunemia (29-34). The complex nature of XLP is the result of aberrant function of multiple different lymphocyte populations that are dependent on the function of SAP and SLAM family members. Studies of SAP-deficient mice have provided insight into the complex manifestations of XLP1 and revealed phenotypes not previously appreciated, including defects in germinal center formation and T cell:B cell interactions (35-37), as well as a lack of Natural killer T (NKT) cells and other innate-like T cell populations that provide a first line defense against infection (38, 39). XLP1 patients exhibit similar defects, including a lack of germinal centers and of NKT cells (38-42). Interestingly, studies in gene-targeted mice have implicated Ly108 in both these phenotypes (37, 43).
The involvement of Ly108 in NKT development suggests that Ly108 actively signals during thymic development (43). To examine Ly108 signaling in the thymus, we evaluated Ly108 protein and phosphorylation status in the intact thymus and in isolated thymocytes. We find that Ly108 is constitutively phosphorylated in the thymus in a SAP and Fyn-dependent manner. Ly108 phosphorylation is rapidly lost upon disaggregation of thymocytes, suggesting that Ly108 is constitutively engaged and dynamically regulated in the thymus. Evaluation of Ly108 message and protein reveal multiple forms that are differentially expressed in lupus-prone and resistant strains of mice. Interestingly, a recently described novel isoform, Ly108-H1, which is uniquely expressed in lupus-resistant C57Bl/6 mice, is not tyrosine phosphorylated despite the presence of an ITSM motif, suggesting that it acts as a decoy-receptor and alters signaling downstream from Ly108.
Materials and Methods
Antibodies
Unconjugated, PE, or biotin conjugated mouse monoclonal antibody directed against Ly108 extracellular domain was from eBioscience, CA. Ly108 polyclonal antisera were generated by Covance Research Products, PA by immunizing rabbits with KLH-conjugated peptides and affinity purified on peptide conjugated-N-Hydroxysuccinimide activated sepharose columns. Pan-Ly108 rabbit polyclonal antibody was generated using an N-terminus-specific peptide CLGESAVLPLKLPAGKIA. Ly108-2 rabbit polyclonal antibody was generated using a C-terminus specific peptide CKKREETVALTGYNQPITLK. αSAP C1 antibody was previously generated by immunizing rabbits with C-terminal peptide GRGPQAPTGRRDSDI(44). αTCR zeta 387 was a gift from L. Samelson (NCI). Antibodies to HuSAP, Lck, Fyn, were from Cell Signaling Technology, MA, anti-phosphotyrosine, 4G10 from Millipore and anti-CD3, and CD4 and CD8 from BD Transduction. Pansorbin (Staph A) was from Calbiochem and Protein A agarose beads were from Santa Cruz Biotechnology, CA.
Mice
Mice were maintained and used in accordance with guidelines of the Institutional Animal Care and Use Committee at NHGRI, NIH. Sh2d1a−/− (SAP-deficient) mice were previously described (44). C57Bl/6 mice were from Jackson Laboratories and MHC Class I/Class II-deficient (B6.129-H2-Ab1tm1Gru B2mtm1Jae) (45)mice were from Taconic Laboratories. Ly108−/− mice were generated from the HGTC-8 C57Bl/6 ES cell line and do not express Ly108, as detected by flow cytometry (manuscript in preparation).
Cell lines and cloning
Ly108-1, Ly108-2 or novel Ly108-H1 isoform were amplified from cDNA made from splenocyte RNA of C57Bl/6 mice using following primers. Ly108 F5′ AACGCGGATCCACCatggctgtctcaagggctccagcacccgac 3′ Ly108-1 R5′ TTCGCTGATATCagagtattcggcctctctggaatgattaac 3′ and Ly108-2 R5′ TTCGCTGATATCggagttatagttgattaaagtgttaaccttc 3′. The Ly108-2 primers also amplified the novel Ly108-H1 isoform. PCR products were cloned in pCTAP (Stratagene) using Eco V/BamHI sites, transfected into the mouse thymoma EL-4 cell line and stable transfectants were selected in G418. Human SH2D1A cloned in a MIGR retroviral vector(46) was used to infect stable cell lines. High Ly108 and SAP expressing clones were sorted on surface Ly108 and GFP levels, cloned by limiting dilution and maintained under G418 selection.
Real time PCR
RNA was extracted from sorted CD4, CD8 and double positive (DP) thymocytes using Trizol. cDNA was made using Applied Biosystems reverse transcription reagents. The following primers and Taqman® probes were designed for real time PCR to detect the three Ly108 isoforms: Ly108-1 common F primer 5′ctcgtccaatgcaggaaatg3′; Ly108-1 reverse primer 5′agatgtgttctccctggatttc3′; and Ly108-1 probe 6FAM-tcattccagagaggccgaatactct-MGBNFQ. Ly108-2 common F primer 5′ctcgtccaatgcaggaaatg3′; Ly108-2 reverse primer 5′aggagttatagttgattaaag3′; and Ly108-2 probe 6FAM-tcattccagagaggaaacagtggc-MGBNFQ Novel Ly108-H1F primer 5′agcacagatggcccagg3′; Novel Ly108-H1 reverse primer 5′aggagttatagttgattaaag3′; and Novel Ly108-H1 probe 6 FAM-aatgcaggaaacagtg-MGBNFQ.
Immunoprecipitation staining and stimulations
Cells were lysed in HNTG lysis buffer (50mM HEPES pH 7.4, 150mM NaCl, 1mM MgCl2, 10%Glycerol, 1mM EDTA) or RIPA buffer (10mM HEPES pH 7.4, 150mM NaCl, 10% glycerol, 1% NP40, 0.5% Sodium deoxycholate, 0.1% SDS) supplemented with protease and phosphatase inhibitor cocktails (Sigma), orthovanadate, and PMSF. For intact organ immunoprecipitations (IP), thymi or lymph nodes from 6-week-old mice were crushed on plastic cell strainers using one ml of complete lysis buffer and transferred to microfuge tubes. Lysis was completed by rocking at 4°C for 30 min and lysates cleared by centrifuging at 15,000 rpm for 15 min. Cleared lysates were incubated with 2.5 μg of Ly108 monoclonal antibody or Ly108-2 polyclonal antibody and incubated at 4°C with protein A beads for 2 hrs. IPs were washed twice with lysis buffer and eluted in 2X SDS loading buffer. For deglycosidase treatment, washed IPs on Protein A beads (Santa Cruz Biotechnology) were treated with PNGase F enzyme (NEB) according to manufacturer’s instructions. For pervanadate treatment, activated sodium pervanadate solution was made by mixing sodium orthovanadate to a final concentration of 30mM in 0.18% hydrogen peroxide solution and incubating in the dark for 15 min. Thymocytes were resuspended in serum free RPMI and incubated for 10 min with 300 μM final concentration of pervanadate. Cells were pelleted and lysed immediately in 1 ml of cold lysis buffer followed by IPs as described. For stimulations followed by IP, 25 × 106 cells were incubated with 10μg/ml final concentration of unconjugated αLy108 and or biotin conjugated αCD3 for 15 min. Stimulations were done by addition of αmouse IgG and or biotin for designated times and stopped by the addition of 2X RIPA followed by adjusting volume with 1X RIPA. Stimulated lysates were cleared and Ly108 was precipitated by the addition of Pansorbin or Protein A for 2 hours. IPs were washed and eluted as described earlier. Samples were run on precast 10%, 4-20% or 18% Tris-Glycine gels (Invitrogen) and transferred to nitrocellulose membranes. True Blot® HRP conjugated αmouse and αrabbit IgG secondary antibodies (Ebiosciences) were used and blots developed by chemilumniscence ECL detection reagents (Amersham™). Films were scanned and protein band intensities were quantitated after background subtraction using image J software. Co-IP and phosphotyrosine intensities were normalized to total Ly108 protein intensities. For staining, 2×106 thymocytes were stained for αCD4 PerCP, αCD8 APC and αLy108 PE antibodies (EBioscience) and read on FACS calibur. Flow cytometry data was examined using FlowJo (Tree Star, Inc.)
Results
Ly108 expression on thymocytes
To evaluate Ly108 in the thymus, we first examined surface Ly108 expression on thymocytes by flow cytometry. We found that Ly108 is highly expressed on thymocytes from C57Bl/6 mice, with greatest expression on double-negative (DN) and double-positive (DP) thymocytes, and down-regulation at the single positive (SP) stage (Figure 1A). Lowest Ly108 expression was seen on the most mature HSAlo SP thymocytes, confirming a reduction in surface Ly108 as thymocytes mature post-selection. (Fig. 1B). Similar results were obtained when Ly108 was evaluated on thymocytes by intracellular staining, suggesting that total Ly108 protein was decreased (Supplemental Fig. S1A). The down regulation during development correlated with reduced Ly108 mRNA at SP stages (Fig. S1B).
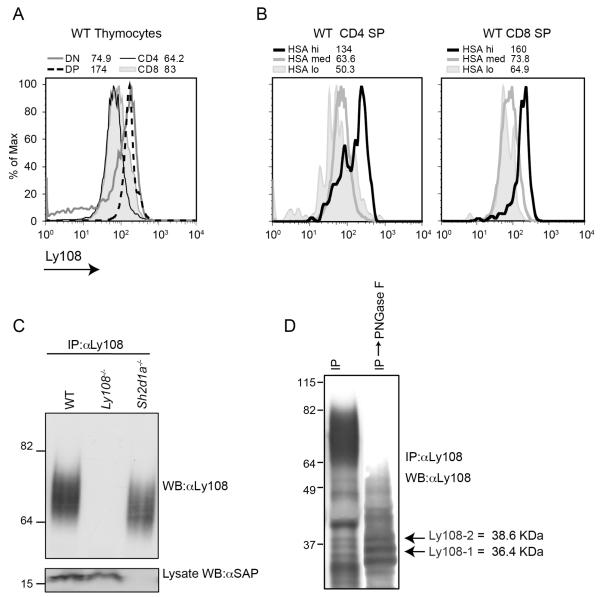
FIGURE 1.
Ly108 expression during thymocyte development. A. WT C57Bl/6 thymocytes were stained for CD4, CD8, HSA and Ly108. Representative histograms and MFIs of Ly108 surface expression on DP, DN, CD4 and CD8 thymocytes are shown from one of five experiments. B. CD4 and CD8 SP thymocytes were gated on HSA (which decreases as cell mature) and Ly108 surface expression examined. C. Ly108 was immunoprecipitated from thymic lysates of WT C57Bl/6, Ly108−/− and Sh2d1a−/−, mice using Ly108 mouse monoclonal antibody and western-blotted with pan-Ly108 rabbit polyclonal antibody. D. Ly108 immunoprecipitates were treated with PNGase F and western-blotted with pan αLy108. Bands corresponding to predicted molecular weights of Ly108-1, Ly108-2 isoforms are indicated. C and D are representative of 6 or more experiments.
To further examine Ly108 protein, we immunoprecipitated Ly108 using a Ly108 mouse monoclonal antibody and western-blotted with a pan-Ly108 rabbit polyclonal antibody directed against the extracellular domain of Ly108. Ly108 protein ran as a diffuse band between 60 and 85 kDa that was present in WT and SAP deficient thymocytes but absent in the immunoprecipitates of Ly108−/− thymocytes, confirming the specificity of the polyclonal antibody (Fig. 1C). Ly108 has multiple spliced message isoforms that are predicted to generate proteins between 35 and 40 kDa. Analysis of Ly108 using the glycosylation prediction software NetNGly 1.0 server (Technical University of Denmark) predicted 7 N-linked glycosylation (N82, N101, N112, N152, N159, N193, and N218), but no O-linked sites in the extracellular domain, potentially contributing to its diffuse slow migration pattern. After treatment of immunoprecipitates with Peptide N Glycosidase F (PNGase F) and immuno-blotting with the pan-Ly108 rabbit polyclonal, the diffusely migrating band now collapsed into several distinct bands, some of which migrated in the predicted molecular weight range of the Ly108 isoforms (Fig. 1D). Thus, Ly108 is a heavily glycosylated protein that is developmentally regulated in thymocytes.
Contact mediated Ly108 phosphorylation in intact thymus
Using isolated thymocytes, Veillette and colleagues have previously shown that Ly108 can be tyrosine phosphorylated in response to stimulation by cross-linking with αLy108 (15). Because Ly108 is a self-ligand that is expressed on most thymocytes, we postulated that Ly108 might be constitutively stimulated in the intact thymus due to thymocyte-thymocyte interactions. To evaluate this hypothesis, we directly lysed intact whole thymi in lysis buffer containing protease and phosphatase inhibitors and evaluated phosphotyrosine on immunoprecipitated Ly108. We compared these immunoprecipitates with those from lysates made from single cell suspension of thymocytes (Fig 2A). Interestingly, Ly108 from intact thymus was heavily tyrosine phosphorylated (Fig. 2B). We also saw evidence for Ly108 tyrosine phosphorylation in directly lysed intact lymph nodes (Fig. S1C). In contrast, when Ly108 was immunoprecipitated from thymocyte single-cell preparations, we did not detect Ly108 tyrosine phosphorylation (Fig. 2B). This loss of tyrosine phosphorylation occurred rapidly (within minutes), as soon as thymocytes were disassociated into single cell preparations. Ly108 phosphorylation could not be recapitulated by merely pelleting the cells together at 37°C (data not shown), but could be reintroduced with pervanadate treatment.
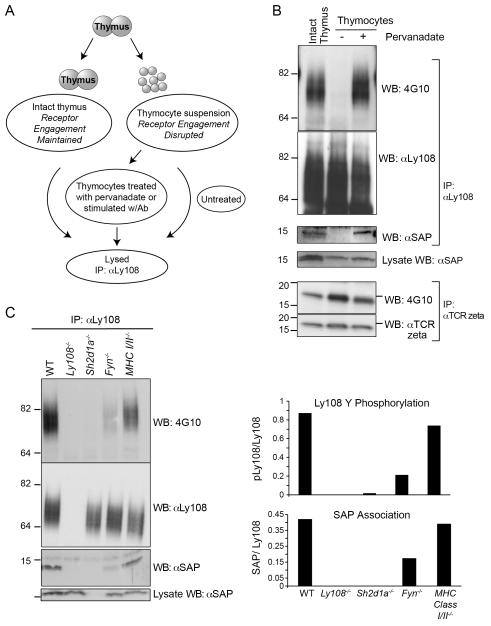
FIGURE 2.
Requirements for Ly108 phosphorylation in the thymus: Contact mediated phosphorylation of Ly108. A. Schematic of lysis procedures. B. Ly108 was immunoprecipitated from (lane 1) intact thymi from C57Bl/6 mice directly lysed; (lane 2) thymocyte suspensions or (lane 3) thymocyte suspensions treated with pervanadate. Lysates were immunoprecipitated with Ly108 monoclonal antibody (top) or αTCR zeta (bottom) and blotted for phosphotyrosine (4G10), Ly108 and SAP (top) or phosphotyrosine and TCR zeta (bottom). C. Ly108 phosphorylation is reduced in absence of Fyn or SAP. Intact thymi from WT, Ly108−/−, Sh2d1a−/−, Fyn−/− and MHC Class I and II deficient mice were lysed, Ly108 immunoprecipitated and immunoblotted for 4G10, Ly108 and SAP. Relative intensities of pLy108/Ly108 and associated SAP/Ly108 are graphed. B and C are representative of 5 or more experiments.
In contrast, TCR zeta, a protein known to be constitutively tyrosine-phosphorylated in the thymus secondary to TCR engagement with self-MHC (47), was still tyrosine phosphorylated in lysates from single cell preparations (Fig. 2B, lower panels), consistent with previous reports that zeta phosphorylation is lost only after several hours of incubation at 37°C (47, 48). Thus, the regulation of Ly108 phosphorylation appears to be both contact-mediated and highly dynamic.
To examine whether Ly108 phosphorylation leads to downstream signaling, we examined SAP association. SAP was found to co-immunoprecipitate with Ly108 in both samples from intact thymus as well as pervanadate treated thymocytes, but not from thymocytes in suspension where Ly108 was not phosphorylated (Fig. 2B). These findings differ from those seen with SLAM, where SAP can also be found in association with unphosphorylated ITSMs (49-52).
Ly108 phosphorylation is reduced in absence of Fyn or SAP
Previous work has demonstrated that SAP and the Src family tyrosine kinase Fyn are required for the induction of Ly108 phosphorylation in response to cross-linking antibody in vitro (15). To evaluate requirements for phosphorylation in vivo, we examined Ly108 phosphorylation in SAP and Fyn-deficient thymi. Ly108 phosphorylation was completely absent in SAP-deficient thymi and was dramatically reduced in Fyn−/− thymi (Fig. 2C). These data indicate the critical requirement of SAP for Ly108 phosphorylation, and implicate Fyn as a key kinase that phosphorylates Ly108 in intact thymi. Nonetheless, the presence of some residual phosphorylation suggests that other tyrosine kinases may also contribute to the Ly108 tyrosine phosphorylation. Parallel to the tyrosine phosphorylation of Ly108, co-immunoprecipitation of SAP was drastically reduced in Fyn−/− thymi, although some association was still consistently detected (Fig. 2C). SAP was not detected in control IPs from Ly108-deficient thymi, demonstrating the specificity of these co-immunoprecipitations.
Induction of Ly108 phosphorylation is potentiated by concomitant α-CD3 stimulation
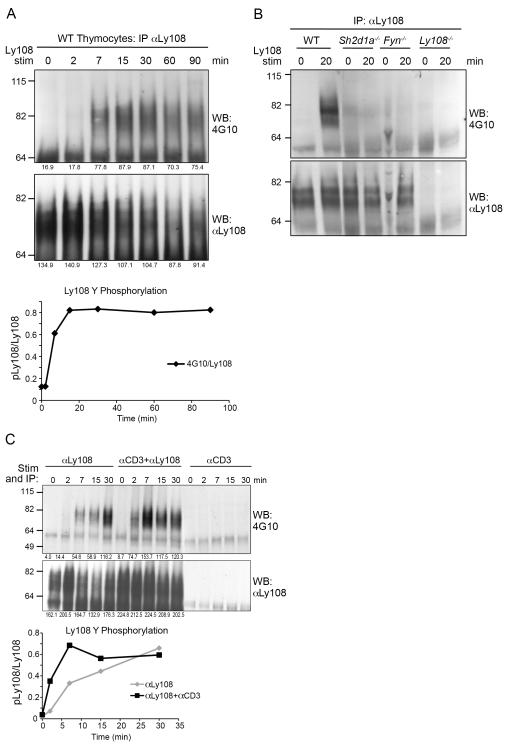
As Ly108 phosphorylation was rapidly lost in single thymocyte suspensions, we evaluated the requirements for induction of Ly108 phosphorylation in cell suspensions using stimulation by cross-linking αLy108 with αmouse IgG. We found that induction of tyrosine phosphorylation of Ly108 occurred with relatively slow kinetics, reaching a peak by 15-30 minutes (Fig. 3A). While the peak tyrosine phosphorylation signal appeared to be reduced by 60 min, this also corresponded with reduced Ly108 protein. Induction of Ly108 tyrosine phosphorylation was severely diminished in the absence of SAP and Fyn (Fig. 3B), as previously reported.
FIGURE 3.
Ly108 rephosphorylation is slow but potentiated by αCD3 engagement in vitro. A. Thymocytes from WT C57Bl/6 were incubated with Ly108 monoclonal antibody and cross linked with α-mouse rabbit IgG for indicated times at 37°C, then lysed followed by immunoprecipitation by addition of Pansorbin A. Immunoprecipitates were probed for phosphotyrosine (4G10) and Ly108. Graph of the time course of Ly108 phosphorylation normalized to Ly108 levels is shown in the lower panel. B. WT, Sh2d1a−/− Fyn−/− and Ly108−/− thymocytes were stimulated and Ly108 immunoprecipitated as in A. C. Thymocytes were isolated and incubated with Ly108 mouse monoclonal antibody and/or biotin conjugated α-CD3ε. Antibodies were cross-linked with αmouse IgG and streptavidin at 37° C for the indicated times and Ly108 immunoprecipitated by addition of Pansorbin, as above. IPs were probed for phosphotyrosine (top) and Ly108 (bottom). Lower panel shows time course of Ly108 phosphorylation normalized to Ly108 levels. A-C are representative of 3 or more experiments.
To determine whether TCR stimulation, which rapidly activates the Src family kinases Lck and Fyn, contributes to Ly108 phosphorylation, we first examined Ly108 phosphorylation in thymi from mice lacking MHC Class I and II, in which the TCR is not engaged. Ly108 phosphorylation was only modestly decreased in thymi from these mice (Fig. 2C). However, as these mice lack mature SP thymocytes, we could not rule out that altered cell populations contributed to any changes in Ly108 phosphorylation. To further examine the impact of TCR stimulation on Ly108 phosphorylation, we cross-linked both Ly108 and the TCR using antibodies directed against Ly108 and CD3ε. We observed that the kinetics of Ly108 stimulation could be potentiated by concomitant TCR stimulation, with the peak Ly108 phosphorylation occurring earlier (7 min) than with Ly108 engagement alone (Fig. 3C and Supplemental Figure S1D). Thus, TCR engagement helps potentiate Ly108 phosphorylation.
Ly108 isoforms in C57Bl/6 mice
Ly108 encodes multiple isoforms that are generated by alternative splicing affecting the C-terminal cytoplasmic tail (13,15). Previous data have demonstrated differential expression of Ly108 isoforms in lupus-prone and lupus-resistant strains of mice (13). To further our understanding of Ly108, we cloned Ly108 cDNA from C57Bl/6 mice (Fig. 4a). Similar to initial descriptions of Ly108, we obtained cDNAs encoding Ly108-1 and Ly108-2, which encode proteins that are identical in the N-terminus, but vary in the C-termini, resulting in different numbers of ITSMs and non-ITSM tyrosine residues. Ly108-1 (36.4 kDa) has 2 classical ITSMs (Y295 and Y319) and a non-ITSM tyrosine (AEYS), whereas Ly108-2 (38.6 kDa) has 2 classical ITSMs (Y295 and Y319) +1 additional non-classical ITSM (Y335) as well as another non-ITSM Y(Y349 NYNS). We further identified a novel Ly108-H1 spliced isoform related to the Ly108-2 isoform, which had deleted exon VII encoding one classical ITSM (Y319) (Fig. 4A and Supplemental Fig. S2). During the course of our studies, this isoform was also reported by the Terhorst group who has designated this species novel Ly108-H1(16).
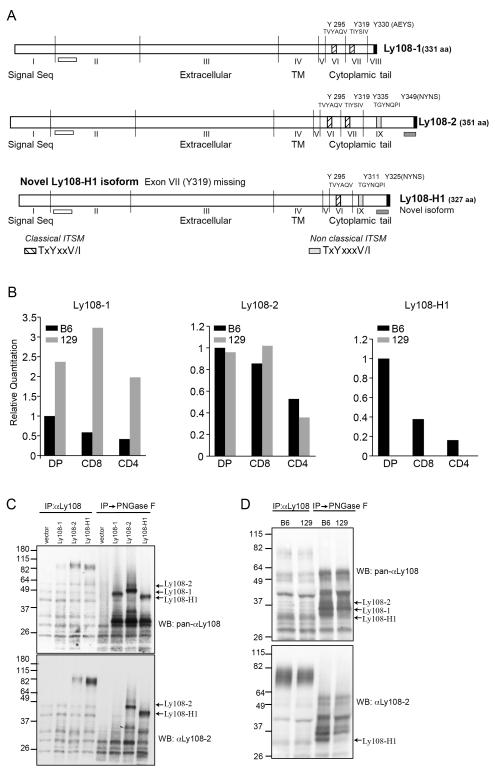
FIGURE 4.
Expression of Ly108 isoforms in C57Bl/6 and 129S6 thymocytes A. Schematic of Ly108 isoforms cloned from C57Bl/6 thymocytes. ITSMs at the carboxy-termini are indicated in hatched boxes and the non-classical ITSM is indicated by a grey box. Non-ITSM tyrosines are in black. The Ly108-1 isoform, has 2 classical ITSMS and 1 non-ITSM tyrosine, Y330. The Ly108-2 isoform, has 2 classical ITSMs, 1 non-classical ITSM (Y335) and 1 non-ITSM tyrosine (Y349). The novel Ly108-H1 isoform, has 1 classical ITSM (Y295), 1 non-classical ITSM (Y311) and 1 non-ITSM tyrosine (Y325), which is equivalent to Y349 of Ly108-2. White bars below exon 2 indicate the extracellular domain peptide used to generate pan-Ly108 polyclonal antibody that detects all the three isoforms. Grey bars below exon 9 indicate the location of the cytoplasmic peptide used to generate αLy108-2, which recognizes Ly108-2 and Ly108-H1, but does not react with Ly108-1. TM: transmembrane domain. B. Real time Taqman probes specific for Ly108-1, 2 and novel Ly108-H1 isoforms were used to detect expression in RNA isolated from sorted and DP and CD4+ and CD8+ SP thymocytes from C57Bl/6 and 129S6 mice. RNA levels were normalized to 18S RNA. Graphs show relative quantification calibrated to C57Bl/6 DP sample for one representative of three experiments. C. Ly108 isoform expressing EL-4 cell lines. Ly108 was immunoprecipitated from stable cell lines expressing individual isoforms using Ly108 monoclonal antibody followed by ± treatment with PNGase F. Ly108 was detected by western-blotting with pan-αLy108 (top panel) or αLy108-2 (bottom panel). Arrows indicate the predicted size of each isoform species including 8 kDa from the TAP-tag. Molecular weights including the TAP-tags are Ly108-1=44.4 kDa, Ly108-2=46.6 kDa and Ly108-H1=43.8 kDa. D. Differential expression of Ly108 isoforms in thymi from C57Bl/6 and 129S6 mice. Ly108 was immunoprecipitated from thymocytes and a portion treated with PNGase F. Ly108 isoforms were detected by immunoblotting with pan-αLy108 (top panel) and αLy108-2 (bottom panel) and are indicated by arrows. C-D are representative of 3 or more experiments.
To evaluate expression of these isoforms, we compared mRNA from C57Bl/6 with 129S6 mice, which express the lupus-prone SLAM haplotype. Examination of these isoforms by quantitative RT-PCR confirmed that 129S6 mice expressed significantly higher levels of Ly108-1, while expressing similar levels of Ly108-2 isoform as C57Bl/6 mice (Fig. 4B). While these finding differ slightly from those of Wandstrat and colleagues (13), who found higher levels of Ly108-2 expressed from the lupus-resistant SLAM haplotype, the primers used in their study would also amplify Ly108-H1. Notably, using isoform-specific Taqman probes, we found that expression of the novel Ly108-H1 was restricted to C57Bl/6 mice that express the lupus-resistant haplotype. Similar results were recently reported by the Terhorst group (16). The lack of this isoform in the lupus-prone haplotype was also confirmed in lupus-prone congenic B6.Sle1b mice that express the lupus-prone SLAM haplotype introgressed onto the C57Bl/6 background (M.D. unpublished data).
To start to evaluate the function of the distinct isoforms, we sub-cloned these isoforms into the pCTAP vector and generated stable clones expressing the individual isoforms in the mouse thymoma cell line EL-4 (Fig. 4C). Similar to the endogenous protein, Ly108 expressed in these cell lines appeared as slowly migrating species, which when deglycosylated, showed distinct bands of sizes corresponding to the predicted fusion protein products. Western blotting with a pan-Ly108 rabbit polyclonal antibody directed against the N-terminus (extracellular domain) of Ly108 confirmed expression of species having the predicted sizes of the tagged proteins: 44.4, 46.6 and 43.8 kDa, corresponding to Ly108-1, Ly108-2 and the novel Ly108-H1 isoform respectively (Fig. 4C, top panel). Of note, this antibody was seen to react more strongly with the deglycosylated species of Ly108.
To further evaluate these distinct isoforms, we also generated rabbit polyclonal antibody directed against the C-termini of Ly108. Although we were unable to obtain a Ly108-1 specific antibody, we were able to obtain a Ly108-2 polyclonal antibody directed against a sequence located in the Ly108-2 isoform. This sequence is also represented in the novel Ly108-H1 isoform (see Fig. 4A for sequence locations). This antibody recognized the Ly108 species in the cell lines expressing Ly108-2 or novel Ly108-H1 but not the Ly108-1 isoform (Fig. 4C, bottom panel).
Examination of immunoprecipitated Ly108 protein from thymocytes treated with PNGase F from C57Bl/6 and 129S6 mice further confirmed that 129S6 mice were largely missing a species that corresponded to the predicted size of the novel Ly108-H1 isoform. Moreover, as predicted, this smaller form reacted with αLy108-2 (Fig. 4D).
Expression and phosphorylation of Ly108 isoforms in C57Bl/6 and 129S6 mice
Given the differences in expression of the splice isoforms in 129S6 and C57Bl/6 mice, we used these two strains to compare the expression and phosphorylation of Ly108. Surprisingly, DP thymocytes from C57Bl/6 mice expressed more than three-fold higher levels of Ly108 on their surface than 129S6 thymocytes (Fig. 5A). Examination of CD4 and CD8 SP cells revealed that 129S6 mice exhibited a broader range of expression, with some cells having very low levels of Ly108, so that there was an even greater difference in the mean fluorescent intensity of Ly108 expression between C57Bl/6 and 129S6. These differences were confirmed by western blotting; while levels of the downstream signaling molecule Fyn appeared equivalent in thymocytes from these mouse strains, Ly108 levels were considerably lower in 129S thymocytes (Fig. 5A). Lower levels of Ly108 expression were also observed in congenic B6.Sle1b mice (16 and M.D. data not shown)
FIGURE 5.
Differential expression and phosphorylation of Ly108 in thymocytes from C57Bl/6 and 129S6 mice. A. Thymocytes from C57Bl/6 and 129S6 mice were stained for surface expression of CD4, CD8 and Ly108 (left). Ly108 was immunoprecipitated from C57Bl/6 and 129S6 thymocytes using αLy108 mouse monoclonal and immunoblotted using pan-αLy108 (top, right). Total Fyn levels in these lysates were compared using αFyn (bottom, right). B. Two examples of Ly108 immunoprecipitated from intact C57Bl/6 and 129S6 thymi using Ly108 monoclonal antibody and immunoblotted for phosphotyrosine, 4G10 (top panel) and pan-Ly108 (bottom panel). Loading of IPs were adjusted to normalize levels of Ly108. Relative intensities of pLy108/Ly108 are graphed. Data are representative of 3 or more experiments.
Despite lower expression levels of Ly108 in 129S6 thymus, we found that Ly108 immunoprecipitated from intact 129S6 thymi was more heavily phosphorylated than that from C57Bl/6 thymi, when normalized for protein levels (Fig. 5B). To evaluate which isoforms contributed to this increase, we used the Ly108 mouse monoclonal antibody (that recognizes all the isoforms) as well as specific Ly108-2 polyclonal antibody to immunoprecipitate Ly108 from intact thymi of C57Bl/6 and 129S6 mice (Fig. 6). After deglycosylation, tyrosine phosphorylated species were compared by western blotting with 4G10 and the pan-αLy108 antibody.
FIGURE 6.
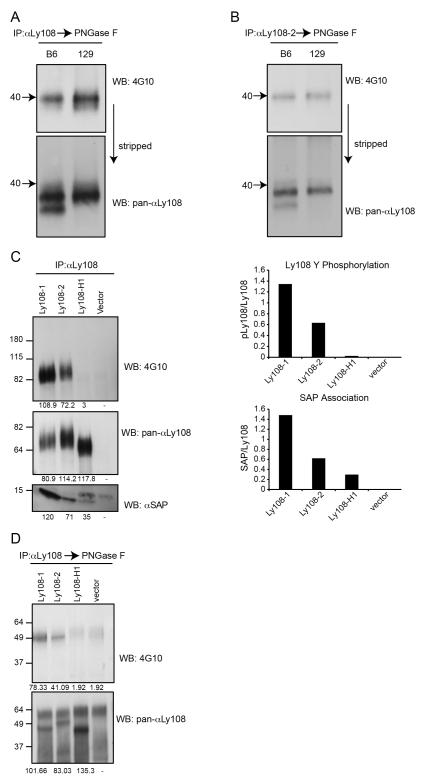
Differential phosphorylation of Ly108 isoforms. A-B. Ly108 was immunoprecipitated from intact thymi of C57Bl/6 and 129S6 mice using Ly108 mouse monoclonal antibody A, or αLy108-2 B, and treated with PNGase F. Western-blots were probed for phosphotyrosine (top) and stripped and re-probed with pan-αLy108 (bottom). Arrows indicate the 40 kDa marker. C. Ly108 was immunoprecipitated using αLy108 mouse monoclonal from pervanadate-treated SAP-expressing EL-4 cell lines expressing the Ly108-1, Ly108-2, novel Ly108-H1 isoforms or the empty vector. Ly108 immunoprecipitates were probed using 4G10 (top), pan-αLy108 (middle panel) and αSAP (lower panel). Relative intensities of pLy108/Ly108 and SAP/Ly108 are graphed. D. Ly108 was immunoprecipitated as in C, followed by treatment with PNGase F and immunoblotted for 4G10 (top) and pan-αLy108 (bottom panel). In parts C and D, the Ly108-H1 and vector control lanes were overloaded to ensure detection of phosphorylated Ly108-H1. Data are representative of 3 or more experiments.
Immunoprecipitation with the Ly108 monoclonal antibody showed primarily one major phosphotyrosine band of approximately 40 kDa that was increased in intensity in 129S6 compared to C57Bl/6 thymi (Fig. 6A top). Reprobing the blot with pan-αLy108 revealed that the tyrosine phosphorylated species migrated more slowly than the bands corresponding to Ly108 isoforms (Fig. 6A bottom), consistent with the slower migration of tyrosine phosphorylated proteins. Notably, there did not appear to be a distinct band that correlated with the phosphorylated novel Ly108-H1 isoform in immunoprecipitates from C57Bl/6 thymi.
Immunoprecipitation with the specific Ly108-2 polyclonal antibody showed a clear single phosphotyrosine band migrating close to 40kDa, that when re-probed with the pan-αLy108, was also found to be higher compared to the Ly108 isoforms. However, in these specific αLy108-2 immunoprecipitations, no difference in phosphorylation between the two strains was observed, despite the presence of the novel Ly108-H1 isoform in C57Bl/6, which was also immunoprecipitated by this antibody (Fig. 6B). Taken together, these results suggested that Ly108-2 phosphorylation was similar between the two strains and that the phosphorylation difference resulted from the differential expression and phosphorylation of the Ly108-1 isoform. Moreover, these results raised the possibility that the novel Ly108-H1 isoform was not efficiently tyrosine-phosphorylated in the intact thymus.
Ly108 isoforms are differentially phosphorylated
In order to evaluate the phosphorylation potential of each isoform in isolation, we took advantage of the Ly108 isoform-expressing EL-4 cell lines that were co-transfected with SAP. As we have been unsuccessful in stimulating Ly108 activity by antibody cross linking in these cell lines, the single-isoform expressing cell lines were treated with pervanadate, followed by Ly108 immunoprecipitation. When adjusted for total amount of protein, the Ly108-1 isoform phosphorylated more efficiently than the Ly108-2 isoform (Fig 6C, top), in agreement with a previous report examining Tac-chimeric versions of Ly108 (15). Consistent with these patterns of phosphorylation, SAP association was also increased with Ly108-1 when compared to Ly108-2 isoform (Fig. 6C bottom). Surprisingly, although the novel Ly108-H1 isoform was expressed, we did not find evidence for its tyrosine phosphorylation, even when overloading the sample to increase its detection (Fig. 6C). Despite the apparent lack of tyrosine phosphorylation, some SAP association could be detected with the novel Ly108-H1 isoform (Fig. 6C bottom).
Evaluation of the deglycosylated immunoprecipitated proteins from cell lines confirmed the results from intact thymi; the Ly108-1 isoform was more heavily phosphorylated and moreover, the migration of the pLy108-1 resembled that of the pLy108-2 isoform (Fig. 6D). Furthermore, we again did not see evidence for tyrosine phosphorylation of the novel Ly108-H1 isoform, even with overloading of this sample. Taken together, these data indicate that Ly108-1 isoform is the most highly phosphorylated isoform of Ly108, whereas the novel Ly108-H1 isoform either fails to be, or is very poorly phosphorylated.
Discussion
Ly108 has been implicated in the development of NKT cells, the regulation of humoral immunity and T and NK cell cytotoxicity. However, despite its involvement in these important immunological processes, little is known about Ly108 regulation and signaling. We present evidence here that Ly108 is dynamically regulated in the thymus, both at the level of expression and tyrosine phosphorylation. We further extend data supporting distinct patterns of phosphorylation of different Ly108 isoforms that are differentially regulated in lupus-prone and resistant strains of mice. Importantly, we provide evidence that the novel Ly108-H1, which is associated with protection from lupus, is not tyrosine phosphorylated in the thymus.
The implication of Ly108 as one of the SLAM family members involved in NKT cell development, suggested that Ly108 is activated during thymic development. Indeed, the widespread expression of Ly108 and its decreased expression in SP thymocytes raises the possibility it maybe involved more broadly in thymocyte differentiation. We found that Ly108 is constitutively phosphorylated in the thymus, suggesting that at least a portion of Ly108 is engaged in the intact organ. We also find evidence of Ly108 phosphorylation in intact lymph nodes, although less pronounced, demonstrating that Ly108 can be found phosphorylated in the periphery as well. Surprisingly, Ly108 phosphorylation appears to be very tightly regulated. As soon as thymocyte interactions were disrupted by making a single cell preparation, Ly108 phosphorylation was lost. This dynamic control of Ly108 phosphorylation was in contrast to phsophorylation of TCR-zeta, which is was relatively stable for several hours after thymic disaggregation.
The rapid loss of Ly108 phosphorylation when cellular contact is lost upon thymic disassociation is a distinctive feature of Ly108 and suggests the rapid action of a phosphatase. Indeed, although SAP has been implicated in the recruitment of the tyrosine kinase Fyn, initial work on SLAM and SAP had suggested that SAP could act as a competitor for negative regulatory molecules such as SHP-1/2 and SHIP. In this regard, it is of interest that Ly108 has been found to bind and activate SHP-1 in the absence of SAP ((22) and R. Zhao, J. Cannons, PLS, manuscript submitted). Although we have not been able to detect co-immunoprecipitation with phosphatases in the thymus, the rapid dephosphorylation of Ly108 suggests that a phosphatase is in close proximity--either directly binding to Ly108 or as part of a complex. Thus, while phosphorylation of Ly108 is dependent on SAP and partially dependent on Fyn, the net phosphorylation status of Ly108 is likely to be the result of a balance of the actions of kinases such as Fyn and phosphatases such as SHP-1.
Although Fyn appears to be the major kinase responsible for Ly108 phosphorylation, we consistently observed some residual phosphorylation in Fyn-deficient thymi, suggesting that other tyrosine kinases also can participate in Ly108 phosphorylation. Such data is also consistent with data from Snow and colleagues, who found that knockdown of Ly108 prevented re-stimulation induced cell death in mature T cells, whereas knockdown of Fyn did not (22). In vitro evidence suggests that Lck can also interact with SAP; thus, Lck may be one such kinase that can also contribute to Ly108 phosphorylation. Consistent with a role for Lck, we find that tyrosine phosphorylation of Ly108 is potentiated by CD3 engagement, which leads to rapid and potent Lck activation. Although we do not know whether CD3 co-engagement leads to the same patterns of phosphorylation of Ly108 tyrosine motifs, such potentiation of Ly108 signaling by TCR stimulation could also be envisioned if the signaling pathways of these two receptors intersect. Recently, it was reported that 2B4 can utilize ITAM motif-containing proteins to influence signaling in NK cells (53). It will be of interest to see whether Ly108 intersects with TCR signaling pathways to cause synergistic downstream effects. Such a potential intersection of signaling would be consistent with a role for Ly108 in affecting T cell development. The kinetics of Ly108 phosphorylation and dephosphorylation also offers some insight into the regulation of Ly108 signaling in the thymus. While the phosphorylation of Ly108 is slow to peak, its dephosphorylation occurs rapidly--it is possible that such a tight regulation of signaling offers stringency to the thymocyte:thymocyte communication process by only allowing longer duration cell interactions to result in productive signaling. It is intriguing to speculate that this feature may contribute to the effects of Ly108 on NKT cell development.
During the course of our study, we often observed a reduction in total Ly108 protein as tyrosine phosphorylation was induced either by pervanadate treatment or αLy108 stimulation (Fig. 3A). This pattern of increased phosphorylation accompanied by reduced protein levels was also observed in intact thymi of 129 mice (Fig. 5A,B). Whether this reduction of Ly108 upon phosphorylation is the result of induced degradation, a general phenomenon by which many signaling pathways are regulated, is an interesting question.
Ly108 has been implicated as a major contributor to the differential effects of SLAM haplotypes on lupus susceptibility and development of antinuclear antibodies (ANAs). Earlier data implicated the increased ratio of Ly108-1 vs. Ly108-2 expression as contributing to the development of ANAs in lupus prone haplotypes. We also observed relatively increased expression of Ly108-1 in thymocytes from 129S6 (having the lupus-prone haplotype) compared to C57Bl/6 mice (having the lupus-resistant haplotype), although we did not observe an accompanying relative decrease in the expression of Ly108-2. However, more recently, expression of a novel spliced Ly108 isoform, Ly108-H1, has been reported, which appears to be expressed solely from the lupus-resistant haplotype. Importantly, expression of this isoform conferred resistance to the development of ANAs, providing evidence for a dominant role for this isoform. We also independently cloned out this isoform and found that it is expressed in C57Bl/6 mice, but not in 129S6 mice which carry the haplotype associated with lupus susceptibility. Furthermore, we have found that expression of novel Ly108-H1 is also lacking in congenic mice carrying the lupus susceptible allele of the SLAM locus introgressed onto C57Bl/6 mice, (B6.Sle1b mice, MD unpublished data). It is of note that the primers used in the previous study would have also amplified the novel Ly108-H1 isoform, likely accounting for the reported relatively increased expression of Ly108-2 in lupus-resistant strains (13).
Our findings support that of Terhorst and colleagues, but provide further insight into the signaling capabilities of the different isoforms of Ly108. Consistent with previous studies using Tac chimeras with the cytoplasmic tails of Ly108 (15), we observe that Ly108-1 is also more heavily phosphorylated than Ly108-2 in thymocytes in vivo. We further provide evidence that the novel Ly108-H1 is not phosphorylated in vivo, nor can phosphorylation be induced when expressed in isolation in cell lines. The varying degree of phosphorylation seen in Ly108 isoforms in vivo, which is recapitulated upon pervanadate treatment, suggests that not all tyrosines in the Ly108 cytoplasmic tail are phosphorylated evenly, and that Ly108 phosphorylation might in fact be a sequential process. The development of phospho-site-specific antibodies for Ly108 may help address this issue, as well as the question of whether Ly108 is differentially phosphorylated under different conditions. Our results further suggest that the novel Ly108-H1 isoform could function as a decoy isoform that may be capable of binding Ly108 on other cells and that contributes to the bulk of total Ly108 protein in the C57Bl/6 thymus without transmitting phosphorylation dependent downstream signals. In this sense, novel Ly108-H1 could help confer lupus protection by mitigating total Ly108 signaling. We, therefore, propose that total Ly108 phosphorylation and signaling differences seen in the thymi of lupus-prone and lupus-resistant strains result from the interplay between expression differences of Ly108-1 (lupus promoting) and novel Ly108-H1 (lupus mitigating) isoforms. Preliminary data suggest that Ly108 can potentiate signals downstream from the TCR (MD, unpublished). Whether novel Ly108-H1 also differentially affects TCR signaling, or affects other downstream outcomes of Ly108 signaling remains an important question.
Supplementary Material
Acknowledgments
The authors would like to thank J.L. Cannons for reagents and mice, M.C. Zhong and A.Veillette for assistance with stimulation protocols, J. Reilley and R. Handon for excellent technical assistance, S. Anderson for assistance with flow cytometry, and J. Fekecs for assistance with graphics.
Abbreviations
- ANA
anti-nuclear antibodies
- DP
double positive
- DN
double negative
- SP
single positive
- IP
immunoprecipitate
- ITSM
immunoreceptor tyrosine-based switch motif
- NKT
Nature Killer T
- PNGase F
Peptide N Glycosidase F
- SH2
Src Homology domain 2
- SHP
SH2 domain containing phosphatase
- SLAM
Signaling Lymphocyte Activation Molecule
- SAP
SLAM associated protein
- XLP
X linked lymphoproliferative disease
Footnotes
This work was supported by intramural funding of the National Human Genome Research Institute.
References
- 1.Mavaddat N, Mason DW, Atkinson PD, Evans EJ, Gilbert RJ, Stuart DI, Fennelly JA, Barclay AN, Davis SJ, Brown MH. Signaling lymphocytic activation molecule (CDw150) is homophilic but self-associates with very low affinity. J Biol Chem. 2000;275:28100–28109. doi: 10.1074/jbc.M004117200. [DOI] [PubMed] [Google Scholar]
- 2.Martin M, Romero X, de la Fuente MA, Tovar V, Zapater N, Esplugues E, Pizcueta P, Bosch J, Engel P. CD84 functions as a homophilic adhesion molecule and enhances IFN-gamma secretion: adhesion is mediated by Ig-like domain 1. J Immunol. 2001;167:3668–3676. doi: 10.4049/jimmunol.167.7.3668. [DOI] [PubMed] [Google Scholar]
- 3.Flaig RM, Stark S, Watzl C. Cutting edge: NTB-A activates NK cells via homophilic interaction. J Immunol. 2004;172:6524–6527. doi: 10.4049/jimmunol.172.11.6524. [DOI] [PubMed] [Google Scholar]
- 4.Cao E, Ramagopal UA, Fedorov A, Fedorov E, Yan Q, Lary JW, Cole JL, Nathenson SG, Almo SC. NTB-A receptor crystal structure: insights into homophilic interactions in the signaling lymphocytic activation molecule receptor family. Immunity. 2006;25:559–570. doi: 10.1016/j.immuni.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 5.Falco M, Marcenaro E, Romeo E, Bellora F, Marras D, Vely F, Ferracci G, Moretta L, Moretta A, Bottino C. Homophilic interaction of NTBA, a member of the CD2 molecular family: induction of cytotoxicity and cytokine release in human NK cells. Eur J Immunol. 2004;34:1663–1672. doi: 10.1002/eji.200424886. [DOI] [PubMed] [Google Scholar]
- 6.Punnonen J, Cocks BG, Carballido JM, Bennett B, Peterson D, Aversa G, de Vries JE. Soluble and membrane-bound forms of signaling lymphocytic activation molecule (SLAM) induce proliferation and Ig synthesis by activated human B lymphocytes. J Exp Med. 1997;185:993–1004. doi: 10.1084/jem.185.6.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romero X, Benitez D, March S, Vilella R, Miralpeix M, Engel P. Differential expression of SAP and EAT-2-binding leukocyte cell-surface molecules CD84, CD150 (SLAM), CD229 (Ly9) and CD244 (2B4) Tissue Antigens. 2004;64:132–144. doi: 10.1111/j.1399-0039.2004.00247.x. [DOI] [PubMed] [Google Scholar]
- 8.Yan Q, Malashkevich VN, Fedorov A, Fedorov E, Cao E, Lary JW, Cole JL, Nathenson SG, Almo SC. Structure of CD84 provides insight into SLAM family function. Proc Natl Acad Sci U S A. 2007;104:10583–10588. doi: 10.1073/pnas.0703893104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cannons JL, Tangye SG, Schwartzberg PL. SLAM family receptors and SAP adaptors in immunity. Annu Rev Immunol. 2011;29:665–705. doi: 10.1146/annurev-immunol-030409-101302. [DOI] [PubMed] [Google Scholar]
- 10.Morel L, Rudofsky UH, Longmate JA, Schiffenbauer J, Wakeland EK. Polygenic control of susceptibility to murine systemic lupus erythematosus. Immunity. 1994;1:219–229. doi: 10.1016/1074-7613(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 11.Tsao BP, Cantor RM, Kalunian KC, Chen CJ, Badsha H, Singh R, Wallace DJ, Kitridou RC, Chen SL, Shen N, Song YW, Isenberg DA, Yu CL, Hahn BH, Rotter JI. Evidence for linkage of a candidate chromosome 1 region to human systemic lupus erythematosus. J Clin Invest. 1997;99:725–731. doi: 10.1172/JCI119217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fossati-Jimack L, Cortes-Hernandez J, Norsworthy PJ, Cook HT, Walport MJ, Botto M. Regulation of B cell tolerance by 129-derived chromosome 1 loci in C57BL/6 mice. Arthritis Rheum. 2008;58:2131–2141. doi: 10.1002/art.23553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wandstrat AE, Nguyen C, Limaye N, Chan AY, Subramanian S, Tian XH, Yim YS, Pertsemlidis A, Garner HR, Jr., Morel L, Wakeland EK. Association of extensive polymorphisms in the SLAM/CD2 gene cluster with murine lupus. Immunity. 2004;21:769–780. doi: 10.1016/j.immuni.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Wang A, Batteux F, Wakeland EK. The role of SLAM/CD2 polymorphisms in systemic autoimmunity. Curr Opin Immunol. 2010;22:706–714. doi: 10.1016/j.coi.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 15.Zhong MC, Veillette A. Control of T lymphocyte signaling by Ly108, a signaling lymphocytic activation molecule family receptor implicated in autoimmunity. J Biol Chem. 2008;283:19255–19264. doi: 10.1074/jbc.M800209200. [DOI] [PubMed] [Google Scholar]
- 16.Keszei M, Detre C, Rietdijk ST, Munoz P, Romero X, Berger SB, Calpe S, Liao G, Castro W, Julien A, Wu YY, Shin DM, Sancho J, Zubiaur M, Morse HC, 3rd, Morel L, Engel P, Wang N, Terhorst C. A novel isoform of the Ly108 gene ameliorates murine lupus. J Exp Med. 2011;208:811–822. doi: 10.1084/jem.20101653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sayos J, Wu C, Morra M, Wang N, Zhang X, Allen D, van Schaik S, Notarangelo L, Geha R, Roncarolo MG, Oettgen H, De Vries JE, Aversa G, Terhorst C. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature. 1998;395:462–469. doi: 10.1038/26683. [DOI] [PubMed] [Google Scholar]
- 18.Shlapatska LM, Mikhalap SV, Berdova AG, Zelensky OM, Yun TJ, Nichols KE, Clark EA, Sidorenko SP. CD150 association with either the SH2-containing inositol phosphatase or the SH2-containing protein tyrosine phosphatase is regulated by the adaptor protein SH2D1A. J Immunol. 2001;166:5480–5487. doi: 10.4049/jimmunol.166.9.5480. [DOI] [PubMed] [Google Scholar]
- 19.Chan B, Lanyi A, Song HK, Griesbach J, Simarro-Grande M, Poy F, Howie D, Sumegi J, Terhorst C, Eck MJ. SAP couples Fyn to SLAM immune receptors. Nat Cell Biol. 2003;5:155–160. doi: 10.1038/ncb920. [DOI] [PubMed] [Google Scholar]
- 20.Chen R, Latour S, Shi X, Veillette A. Association between SAP and FynT: Inducible SH3 domain-mediated interaction controlled by engagement of the SLAM receptor. Mol Cell Biol. 2006;26:5559–5568. doi: 10.1128/MCB.00357-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Latour S, Gish G, Helgason CD, Humphries RK, Pawson T, Veillette A. Regulation of SLAM-mediated signal transduction by SAP, the X-linked lymphoproliferative gene product. Nat Immunol. 2001;2:681–690. doi: 10.1038/90615. [DOI] [PubMed] [Google Scholar]
- 22.Snow AL, Marsh RA, Krummey SM, Roehrs P, Young LR, Zhang K, van Hoff J, Dhar D, Nichols KE, Filipovich AH, Su HC, Bleesing JJ, Lenardo MJ. Restimulation-induced apoptosis of T cells is impaired in patients with X-linked lymphoproliferative disease caused by SAP deficiency. J Clin Invest. 2009;119:2976–2989. doi: 10.1172/JCI39518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bottino C, Falco M, Parolini S, Marcenaro E, Augugliaro R, Sivori S, Landi E, Biassoni R, Notarangelo LD, Moretta L, Moretta A. NTB-A [correction of GNTB-A], a novel SH2D1A-associated surface molecule contributing to the inability of natural killer cells to kill Epstein-Barr virus-infected B cells in X-linked lymphoproliferative disease. J Exp Med. 2001;194:235–246. doi: 10.1084/jem.194.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parolini S, Bottino C, Falco M, Augugliaro R, Giliani S, Franceschini R, Ochs HD, Wolf H, Bonnefoy JY, Biassoni R, Moretta L, Notarangelo LD, Moretta A. X-linked lymphoproliferative disease. 2B4 molecules displaying inhibitory rather than activating function are responsible for the inability of natural killer cells to kill Epstein-Barr virus-infected cells. J Exp Med. 2000;192:337–346. doi: 10.1084/jem.192.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castro AG, Hauser TM, Cocks BG, Abrams J, Zurawski S, Churakova T, Zonin F, Robinson D, Tangye SG, Aversa G, Nichols KE, de Vries JE, Lanier LL, O’Garra A. Molecular and functional characterization of mouse signaling lymphocytic activation molecule (SLAM): differential expression and responsiveness in Th1 and Th2 cells. J Immunol. 1999;163:5860–5870. [PubMed] [Google Scholar]
- 26.Li C, Iosef C, Jia CY, Han VK, Li SS. Dual functional roles for the X-linked lymphoproliferative syndrome gene product SAP/SH2D1A in signaling through the signaling lymphocyte activation molecule (SLAM) family of immune receptors. J Biol Chem. 2003;278:3852–3859. doi: 10.1074/jbc.M206649200. [DOI] [PubMed] [Google Scholar]
- 27.Coffey AJ, Brooksbank RA, Brandau O, Oohashi T, Howell GR, Bye JM, Cahn AP, Durham J, Heath P, Wray P, Pavitt R, Wilkinson J, Leversha M, Huckle E, Shaw-Smith CJ, Dunham A, Rhodes S, Schuster V, Porta G, Yin L, Serafini P, Sylla B, Zollo M, Franco B, Bolino A, Seri M, Lanyi A, Davis JR, Webster D, Harris A, Lenoir G, de St Basile G, Jones A, Behloradsky BH, Achatz H, Murken J, Fassler R, Sumegi J, Romeo G, Vaudin M, Ross MT, Meindl A, Bentley DR. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nat Genet. 1998;20:129–135. doi: 10.1038/2424. [DOI] [PubMed] [Google Scholar]
- 28.Nichols KE, Harkin DP, Levitz S, Krainer M, Kolquist KA, Genovese C, Bernard A, Ferguson M, Zuo L, Snyder E, Buckler AJ, Wise C, Ashley J, Lovett M, Valentine MB, Look AT, Gerald W, Housman DE, Haber DA. Inactivating mutations in an SH2 domain-encoding gene in X-linked lymphoproliferative syndrome. Proc Natl Acad Sci U S A. 1998;95:13765–13770. doi: 10.1073/pnas.95.23.13765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bar RS, DeLor CJ, Clausen KP, Hurtubise P, Henle W, Hewetson JF. Fatal infectious mononucleosis in a family. N Engl J Med. 1974;290:363–367. doi: 10.1056/NEJM197402142900704. [DOI] [PubMed] [Google Scholar]
- 30.Harada S, Sakamoto K, Seeley JK, Lindsten T, Bechtold T, Yetz J, Rogers G, Pearson G, Purtilo DT. Immune deficiency in the X-linked lymphoproliferative syndrome. I. Epstein-Barr virus-specific defects. J Immunol. 1982;129:2532–2535. [PubMed] [Google Scholar]
- 31.Provisor AJ, Iacuone JJ, Chilcote RR, Neiburger RG, Crussi FG. Acquired agammaglobulinemia after a life-threatening illness with clinical and laboratory features of infectious mononucleosis in three related male children. N Engl J Med. 1975;293:62–65. doi: 10.1056/NEJM197507102930202. [DOI] [PubMed] [Google Scholar]
- 32.Purtilo DT, Cassel CK, Yang JP, Harper R. X-linked recessive progressive combined variable immunodeficiency (Duncan’s disease) Lancet. 1975;1:935–940. doi: 10.1016/s0140-6736(75)92004-8. [DOI] [PubMed] [Google Scholar]
- 33.Booth C, Gilmour KC, Veys P, Gennery AR, Slatter MA, Chapel H, Heath PT, Steward CG, Smith O, O’Meara A, Kerrigan H, Mahlaoui N, Cavazzana-Calvo M, Fischer A, Moshous D, Blanche S, Pachlopnick-Schmid J, Latour S, de Saint-Basile G, Albert M, Notheis G, Rieber N, Strahm B, Ritterbusch H, Lankester A, Hartwig NG, Meyts I, Plebani A, Soresina A, Finocchi A, Pignata C, Cirillo E, Bonanomi S, Peters C, Kalwak K, Pasic S, Sedlacek P, Jazbec J, Kanegane H, Nichols KE, Hanson IC, Kapoor N, Haddad E, Cowan M, Choo S, Smart J, Arkwright PD, Gaspar HB. X-linked lymphoproliferative disease due to SAP/SH2D1A deficiency: a multicenter study on the manifestations, management and outcome of the disease. Blood. 2011;117:53–62. doi: 10.1182/blood-2010-06-284935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pachlopnik Schmid J, Canioni D, Moshous D, Touzot F, Mahlaoui N, Hauck F, Kanegane H, Lopez-Granados E, Mejstrikova E, Pellier I, Galicier L, Galambrun C, Barlogis V, Bordigoni P, Fourmaintraux A, Hamidou M, Dabadie A, Le Deist F, Haerynck F, Ouachee-Chardin M, Rohrlich P, Stephan JL, Lenoir C, Rigaud S, Lambert N, Milili M, Schiff C, Chapel H, Picard C, de Saint Basile G, Blanche S, Fischer A, Latour S. Clinical similarities and differences of patients with X-linked lymphoproliferative syndrome type 1 (XLP-1/SAP deficiency) versus type 2 (XLP-2/XIAP deficiency) Blood. 2011;117:1522–1529. doi: 10.1182/blood-2010-07-298372. [DOI] [PubMed] [Google Scholar]
- 35.Crotty S, Kersh EN, Cannons J, Schwartzberg PL, Ahmed R. SAP is required for generating long-term humoral immunity. Nature. 2003;421:282–287. doi: 10.1038/nature01318. [DOI] [PubMed] [Google Scholar]
- 36.Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 2008;455:764–769. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cannons JL, Qi H, Lu KT, Dutta M, Gomez-Rodriguez J, Cheng J, Wakeland EK, Germain RN, Schwartzberg PL. Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84. Immunity. 2010;32:253–265. doi: 10.1016/j.immuni.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung B, Aoukaty A, Dutz J, Terhorst C, Tan R. Signaling lymphocytic activation molecule-associated protein controls NKT cell functions. J Immunol. 2005;174:3153–3157. doi: 10.4049/jimmunol.174.6.3153. [DOI] [PubMed] [Google Scholar]
- 39.Pasquier B, Yin L, Fondaneche MC, Relouzat F, Bloch-Queyrat C, Lambert N, Fischer A, de Saint-Basile G, Latour S. Defective NKT cell development in mice and humans lacking the adapter SAP, the X-linked lymphoproliferative syndrome gene product. J Exp Med. 2005;201:695–701. doi: 10.1084/jem.20042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma CS, Pittaluga S, Avery DT, Hare NJ, Maric I, Klion AD, Nichols KE, Tangye SG. Selective generation of functional somatically mutated IgM+CD27+, but not Ig isotype-switched, memory B cells in X-linked lymphoproliferative disease. J Clin Invest. 2006;116:322–333. doi: 10.1172/JCI25720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma CS, Hare NJ, Nichols KE, Dupre L, Andolfi G, Roncarolo MG, Adelstein S, Hodgkin PD, Tangye SG. Impaired humoral immunity in X-linked lymphoproliferative disease is associated with defective IL-10 production by CD4+ T cells. J Clin Invest. 2005;115:1049–1059. doi: 10.1172/JCI23139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nichols KE, Hom J, Gong SY, Ganguly A, Ma CS, Cannons JL, Tangye SG, Schwartzberg PL, Koretzky GA, Stein PL. Regulation of NKT cell development by SAP, the protein defective in XLP. Nat Med. 2005;11:340–345. doi: 10.1038/nm1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Griewank K, Borowski C, Rietdijk S, Wang N, Julien A, Wei DG, Mamchak AA, Terhorst C, Bendelac A. Homotypic interactions mediated by Slamf1 and Slamf6 receptors control NKT cell lineage development. Immunity. 2007;27:751–762. doi: 10.1016/j.immuni.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Czar MJ, Kersh EN, Mijares LA, Lanier G, Lewis J, Yap G, Chen A, Sher A, Duckett CS, Ahmed R, Schwartzberg PL. Altered lymphocyte responses and cytokine production in mice deficient in the X-linked lymphoproliferative disease gene SH2D1A/DSHP/SAP. Proc Natl Acad Sci U S A. 2001;98:7449–7454. doi: 10.1073/pnas.131193098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grusby MJ, Auchincloss H, Jr., Lee R, Johnson RS, Spencer JP, Zijlstra M, Jaenisch R, Papaioannou VE, Glimcher LH. Mice lacking major histocompatibility complex class I and class II molecules. Proc Natl Acad Sci U S A. 1993;90:3913–3917. doi: 10.1073/pnas.90.9.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cannons JL, Yu LJ, Hill B, Mijares LA, Dombroski D, Nichols KE, Antonellis A, Koretzky GA, Gardner K, Schwartzberg PL. SAP regulates T(H)2 differentiation and PKC-theta-mediated activation of NF-kappaB1. Immunity. 2004;21:693–706. doi: 10.1016/j.immuni.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 47.van Oers NS, Tao W, Watts JD, Johnson P, Aebersold R, Teh HS. Constitutive tyrosine phosphorylation of the T-cell receptor (TCR) zeta subunit: regulation of TCR-associated protein tyrosine kinase activity by TCR zeta. Mol Cell Biol. 1993;13:5771–5780. doi: 10.1128/mcb.13.9.5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakayama T, Singer A, Hsi ED, Samelson LE. Intrathymic signalling in immature CD4+CD8+ thymocytes results in tyrosine phosphorylation of the T-cell receptor zeta chain. Nature. 1989;341:651–654. doi: 10.1038/341651a0. [DOI] [PubMed] [Google Scholar]
- 49.Li SC, Gish G, Yang D, Coffey AJ, Forman-Kay JD, Ernberg I, Kay LE, Pawson T. Novel mode of ligand binding by the SH2 domain of the human XLP disease gene product SAP/SH2D1A. Curr Biol. 1999;9:1355–1362. doi: 10.1016/s0960-9822(00)80080-9. [DOI] [PubMed] [Google Scholar]
- 50.Poy F, Yaffe MB, Sayos J, Saxena K, Morra M, Sumegi J, Cantley LC, Terhorst C, Eck MJ. Crystal structures of the XLP protein SAP reveal a class of SH2 domains with extended, phosphotyrosine-independent sequence recognition. Mol Cell. 1999;4:555–561. doi: 10.1016/s1097-2765(00)80206-3. [DOI] [PubMed] [Google Scholar]
- 51.Finerty PJ, Jr., Muhandiram R, Forman JD. Side-chain dynamics of the SAP SH2 domain correlate with a binding hot spot and a region with conformational plasticity. J Mol Biol. 2002;322:605–620. doi: 10.1016/s0022-2836(02)00803-3. [DOI] [PubMed] [Google Scholar]
- 52.Hwang PM, Li C, Morra M, Lillywhite J, Muhandiram DR, Gertler F, Terhorst C, Kay LE, Pawson T, Forman-Kay JD, Li SC. A “three-pronged” binding mechanism for the SAP/SH2D1A SH2 domain: structural basis and relevance to the XLP syndrome. EMBO J. 2002;21:314–323. doi: 10.1093/emboj/21.3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bida AT, Upshaw Neff JL, Dick CJ, Schoon RA, Brickshawana A, Chini CC, Billadeau DD. 2B4 utilizes ITAM-containing receptor complexes to initiate intracellular signaling and cytolysis. Mol Immunol. 2011;48:1149–1159. doi: 10.1016/j.molimm.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.