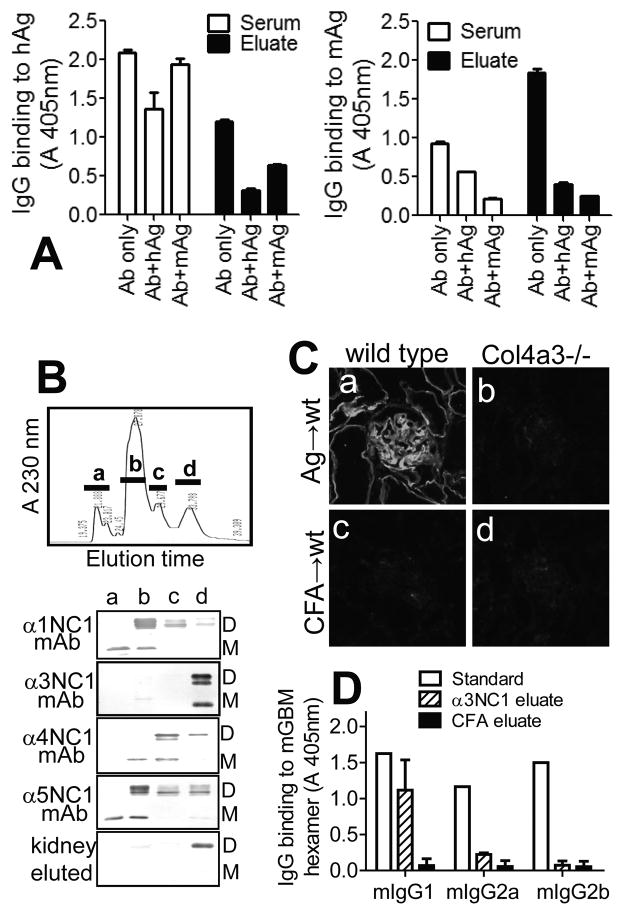
Figure 7. Specificity of kidney-bound mIgG autoAbs.
A. ELISA shows the binding of serum (open bars) and kidney-eluted (closed bars) mIgG Abs to immobilized rh-α3NC1 (hAg, left) and total NC1 hexamers from mouse GBM (mAg, right), and the inhibition of this binding by hAg (5 μg/ml) or mAg (50 μg/ml) in solution. B. NC1 domains from mouse GBM were separated by reverse-phase HPLC on a C18 column (top), and the composition of the major peaks (a–d) was analyzed by Western blotting with chain-specific mAbs (bottom). Kidney-eluted mIgG autoAbs reacted only with the fraction containing murine α3NC1 (M=monomers; D=dimers). C. Indirect IF staining shows the binding of mIgG eluted from the kidneys of α3NC1-immunized mice (a–b) or control mice (c–d) to kidney cryostat sections from wild type mice (a,c) and Col4a3−/− Alport mice (b,d). D. ELISA analysis of subclasses of mIgG eluted from kidneys at ~10 weeks after immunization with rh-α3NC1 (diagonal lines) or CFA (solid bars) showed the prevalence of mIgG1 autoAbs binding to immobilized mouse GBM NC1 hexamers. Anti-NC1 mAbs TF51, TF90 and TF86 (1 μg/ml each) were used as standards for mIgG1, mIgG2a and mIgG2b, respectively (open bars). A similar distribution of mIgG subclasses was found for kidney-eluted autoAbs binding to rh-α3NC1 (not shown).