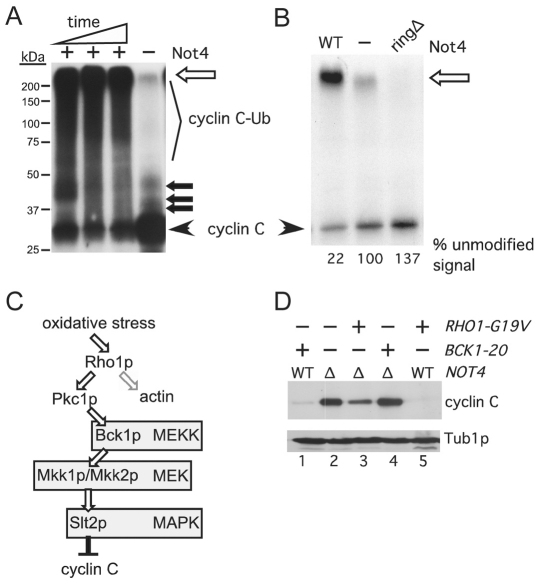
Fig. 2.
Not4p ubiquitylates cyclin C in vitro. (A) In vitro ubiquitylation assays were performed as described in the Materials and Methods. The timepoints were 15, 30 and 90 minutes with a constant concentration of Not4p. The ubiquitylated (cyclin C-Ub) and unmodified (cyclin C) species are indicated. The open arrow indicates the boundary between the resolving and stacking gel. Closed arrows indicate predicted mono-, bi-, and tri-ubiquitylated cyclin C species. Molecular mass markers are indicated. (B) The experiment described in A was repeated with either Not4p (WT), Not4pringΔ (ringΔ) or no ligase (−) added. These reactions were carried out to completion (120 minutes). This gel was exposed to film for a shorter time to visualize the reduction in unmodified cyclin C in the Not4p reaction compared with that for Not4pringΔ. The unmodified signal was quantified by phosphorimaging with the no ligase control set at 100%. (C) Diagram of the cell-wall-integrity MAPK signal transduction pathway. Bifurcation of Rho1p signaling towards Pkc1p and actin organization is indicated. (D) Steady-state myc–cyclin-C levels were determined in non-stressed mid-log NOT4 (WT) or not4Δ (Δ) cultures expressing activated alleles of BCK1 or RHO1, as indicated. Tub1p levels were monitored as a loading control.