Abstract
Skin cancer involving the scalp is a common malignancy in the “sun belt areas of the United States.” Most early lesions are well managed by primary care physicians and dermatologists. Occasionally we encounter basal cell, squamous cell, and rarely Merkel cell carcinomas that have failed local therapy and present with large tumors invading full thickness scalp, calvarium, and even underlying dura. We describe our experience with 52 such tumors and illustrate their resections and reconstruction. For full thickness lesions we generally do a wide field resection of skin and underlying calvarium followed by dural resection. Reconstruction is usually with dural replacement, calvarial reconstruction with titanium mesh, and cutaneous reconstruction with a musculocutaneous free flap or muscular free flap with an overlying skin graft. Complications, survival rates, and recurrence rates will be presented.
Keywords: Skull base, skin cancer, otolaryngology, surgery, scalp carcinoma, free flap, dural invasion
Carcinoma of the scalp is a common disease in the so-called “sun belt” regions of the United States. Squamous cell carcinomas, basal cell carcinoma, malignant melanoma, and malignant fibrous histiocytoma comprise the main bulk of these malignancies. The commonest etiological factor is the exposure to ultraviolet irradiation especially that in the UV B range (290 to 320 nm). This leads to genetic mutations by the promotion of pyrimidine dimers in the DNA of cutaneous cells which in turn lead to inactivation of tumor suppressor genes, thus promoting maignancy.1,2,3 UVB also may act as a suppressor of the local immune response to malignancies as well.1,4
These tumors are commonest in bald Caucasian males especially with fair hair and blue eyes with a Fitzpatrick classification5 of I and II. Patients suffering disorders producing immune suppression are particularly vulnerable to actinic-induced skin malignancy. The metastatic rate in these individuals is much higher as well. The squamous and basal cell lesions that make up the bulk of the tumors usually begin in an actinic keratosis. Most lesions are well treated in the actinic keratosis stage by liquid nitrogen, electrodessication and curettage, or topical chemotherapy such as topical 5-flurouracil.
Over time a large percentage of actinic lesions will degenerate into cutaneous squamous cell or basal cell carcinomas. The probability of developing a squamous cell carcinoma in an actinic keratosis is quoted to be from 8 to 10%1 to 12 to 25%.6,7 Cyclin D1, a protein that often precedes cell development from the resting phase of the cell cycle to that of DNA production is overexpressed in actinic keratosis and may be a preliminary event in the development of malignancy.8,9
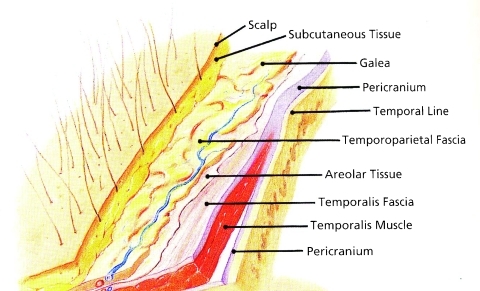
The scalp is comprised of five principal layers as depicted in the acronym SCALP: Skin, subcutaneous tissue, aponeurosis, loose areolar tissue, and periosteum (Fig. 1). Lesions limited to the skin only are most commonly seen and usually dealt with effectively by the dermatologist using MOHS micrographic excision. This technique developed in the 1930s by Dr. Frederick Mohs10 was modified by Dr. Ted Tromovich11 in the 1970s changing the in situ zinc chloride fixation technique of Mohs which was quite painful for the patient to the fresh frozen technique still used today. The technique been expanded to the management of tumors with deeper penetration but this is unfortunately accompanied by a higher degree of local recurrence.
Figure 1.
Layers of the scalp. (Reproduced from Cheney ML. Facial Plastic Surgery: Plastic and Reconstructive. Baltimore: Williams and Wilkins Publishers; 1997.)
The skin lesions that are the most difficult to excise are those that penetrate beyond the galea and extend to the periosteum, calvarium, and intracranially to the dura and underlying brain. A skull base team comprised of a head and neck surgeon, neurosurgeon, and sometimes plastic surgeon is oftentimes necessary to clear the tumor and provide adequate reconstruction.
MATERIALS AND METHODS
This study was conducted using a prospectively acquired database, approved by the UC Davis IRB, gathered between January 1999 and October 2008. It contains the records of 4200 patients who have had head and neck tumors. At the University of California Center for Skull Base Surgery there have been 52 patients who were seen and qualify as extensive carcinomas of the scalp. They were reviewed at the end of the study period. Although all patients had what would be considered as deep invasion of the scalp, in that adequate excision required full thickness scalp excision through the galea to periosteum, a classification system was devised to separate the patients into three classes dependent on the depth of invasion and the necessary surgery required for complete tumor extirpation (Table 1). As there were 48 of the original group of 52 patients who presented with deeply invasive scalp tumors who had surgery at our institution, the analysis of invasive type was limited to the 48.
Table 1.
Classification of Extensive Scalp Carcinoma Based on Tissues Resected
| Superficial | Intermediate | Deep |
|---|---|---|
| Scalp | Scalp | Scalp |
| Galea | Galea | Galea |
| ± periosteum | Periosteum | Periosteum |
| Outer table calvarium | Full thickness calvarium | |
| Dura |
The patients who had what were classified as a “superficial” type of lesion required full thickness scalp excision at least to the galea. Periosteal excision was done only when the galea was penetrated. Individuals in this group may have had resection of the outer table layer of the calvarium to the diploe only to enhance the take of the split-thickness skin graft used for reconstruction. There were 17 patients in the “superficial” resection group.
Those who had “intermediate” resections had invasion of periosteum, were stuck to the bone so that an outer layer resection of the calvarium to the diploe was necessary to achieve tumor clearance. There were 20 patients in the “intermediate” resection category. The least number of patients, 11 of the cohort had the deepest invasion which required full thickness craniectomy usually accompanied by dural excision. These were in the deep resection group (Fig. 2). None of the patients in this series had brain invasion. Two cases had an attempt at intermediate resection taking only the outer calvarium but later when they recurred locally had to be converted to the more major, deep resection.
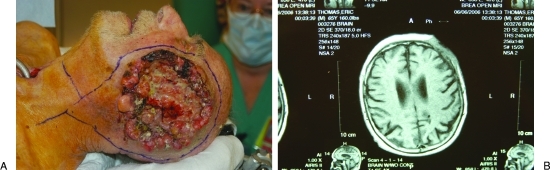
Figure 2.
(A) Patient following multiple Moh's micrographic, as well as, conventional resection for squamous cell carcinoma of the scalp now penetrating the calvarium and infiltrating the dura. (B) MRI showing carcinomatous extension of scalp carcinoma through calvarium and infiltrating dura.
SURGICAL TECHNIQUE
Superficial Resection
The superficial resection entails removal of the full thickness scalp either leaving the periosteum or taking it if the galeal invasion is deep to get a satisfactory margin of resection. Depending on tumor type, a 1- to 2-cm margin of normal appearing scalp is incised down to the underlying periosteum. Any area of surrounding erythema that persists after a course of antibiotics, given when secondary infection exists, is considered to represent dermal extension of tumor and is skirted at times by a margin of as much as 3 cm.
Numerous regularly spaced strips of scalp 2 to 3 mm in width are taken around the circumference of the incision and sent for frozen section analysis. If positive, a further 1-cm margin of scalp is taken and an additional frozen section sent. Raney clips are placed and bipolar cautery employed to secure hemostasis (Fig. 3).
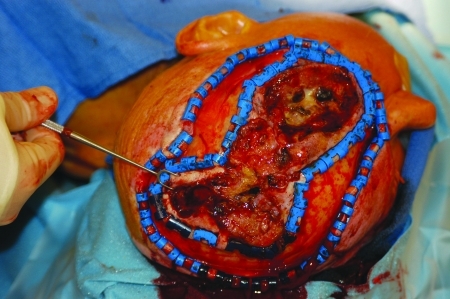
Figure 3.
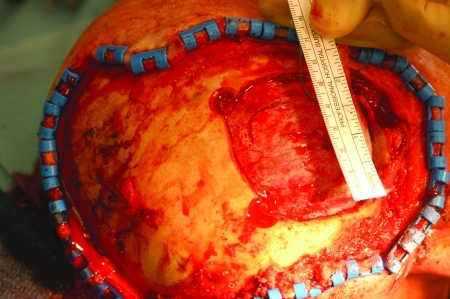
Patient with massive squamous cell carcinoma of the scalp with invasion of calvarium, dura, temporal bone, parotid gland facial nerve, and facial and neck skin.
The layers of the scalp are separated from the periosteum and the specimen submitted for histological examination. Small samples of underlying periosteum are sent for frozen section analysis. If galeal invasion is in close approximation to the periosteum this layer is then removed. The outer layer of calvarium is resected down to the diploe so that a split thickness graft can be used for coverage.
Reconstruction in most instances is with a split-thickness skin graft. Flaps are avoided because of the danger of covering up possible early recurrence which can often be easily managed by limited further local excision. In some instances the luxury of a skin graft cannot be afforded because the patient has been previously irradiated. In these cases local scalp flaps such as those designed by Orticochea12 can be rotated into the defect or the area can be closed with a vascularized free flap. The free flap is employed if the patient had been formerly treated with irradiation therapy
Intermediate Resection
Preoperative evidence of outer cortical bone irregularity on CT scanning is suggestive of outer table invasion. Pitting and irregularity of the calvarial bone on direct visualization at the time of surgery are highly suspicious signs of malignant invasion of the outer cortex. The bone is removed by either splitting the outer cortex with a sagittal cutting saw and osteotomes, using the diploe as the resection plane, or by drilling the outer table with a high speed drill. We usually use a large cutting burr and cut away the outer cortex then send samples of diploe for frozen section analysis. If any of the diploic area returns positive for tumor then the procedure is turned into a “deep resection” and full thickness bony excision is undertaken. The disadvantage of using a drill and not excising a single layer of outer calvarium is not having the bony specimen for permanent section analysis. In our experience with skull base surgery the drilling approach has proven to be highly successful in excising bone with tumor invasion.13,14
Deep Resection
The procedure begins with a full thickness incision through all the layers of the scalp, through periosteum and down to calvarial bone. Depending on tumor type a 1- to 2-cm margin of healthy appearing scalp is taken and multiple superficial and deep margins are sent to pathology for frozen section analysis. Deep margins of galea and periosteum wide of the resection are taken because of the propensity of some tumors to spread along their facial planes. Raney clips and bipolar cautery are used for hemostasis (Fig. 4).
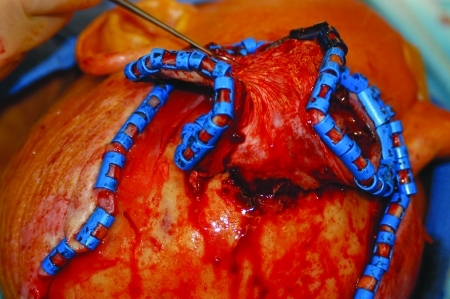
Figure 4.
Scalp incised through periosteum. Raney clips are used for hemostasis.
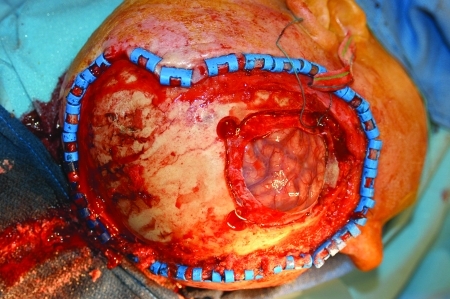
The edge of bony involvement is determined by gently dissecting the periosteum with a periosteal elevator from the bone until the tumor appears to be adherent. Any stippling of the bone must be construed as tumor invasion. A burr hole is made and the craniotome used to take ~1 cm of healthy bone around the area of obvious tumor penetration (Fig. 5). Frozen sections of the diploe are sent to establish the integrity of the bony margins.
Figure 5.
Calvarial resection.
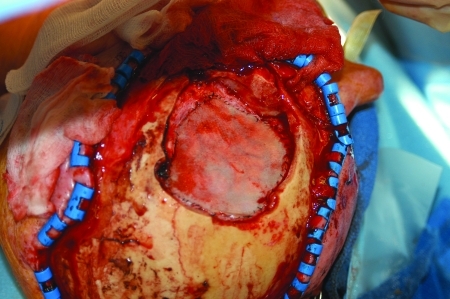
The area of dural invasion is addressed next. Fortunately the dura is highly resistant to both lateral tumor spread and to penetration beyond its inner layer to the underlying brain. Usually a 5-mm margin is all that is required for dural clearance15 (Fig. 6). Margins again are sampled to establish tumor-free margins. One of the major problems in dealing with scalp carcinoma that involves dura is spread to the adjacent bridging veins. This is a particular problem in the vicinity of the superior sagittal sinus. This sinus, if taken posteriorly to the coronal suture, carries with it a high incidence of fatality. Similarly the taking of other bridging veins at this site can produce major problems with venous drainage from the cerebral cortex. The danger is exemplified by the so-called vein of Labbé which often provides the principal drainage conduit from the temporal lobe of the brain to the lateral venous sinus. If the superior vein of Trolard is atretic, the vein of Labbé will then constitute the only venous drainage from the involved hemisphere; in such circumstances taking of the vein will likely result in death of the patient. Those scalp lesions that are more inferiorly located in the temporal scalp and close to the ear may put this bridging vein in jeopardy. Careful analysis of the MRI, standard CT scans, positron emission tomography-CT, and MRA or conventional angiography, if necessary, must be done in hopes of assessing this risk. In two of our patients the resection had to be aborted due to the number of bridging veins involved in tumor connecting to the superior sagittal sinus in one and direct penetration of the superior sagittal sinus in the other.
Figure 6.
Dural resection.
None of our patients had brain invasion. Resection of brain involvement by malignancy is a controversial issue. In our experience of all skull base surgery cases at all sites we have attempted a complete resection of involved noneloquent brain invaded by malignant disease, achieving negative margins in 23 patients. The margin of safe resection is determined by the neurosurgical member of the team as is the extent to which the resection can be performed without significant neurological impairment. The 5-year tumor-free survival rate in our series of all skull base tumors with brain resection is 25.7%.
Reconstruction begins with dural reconstruction (Fig. 7). This is achieved by the use of grafts of either temporalis fascia, if available, or fascia lata. Bovine pericardium or AlloDerm (Brachburg, NJ) as well as other alloplastic dural substitutes may be employed although the alloplasts are not advisable in a previously irradiated field. A concerted effort is made to establish a water-tight seal.
Figure 7.
Dural reconstruction with Bovine pericardium.
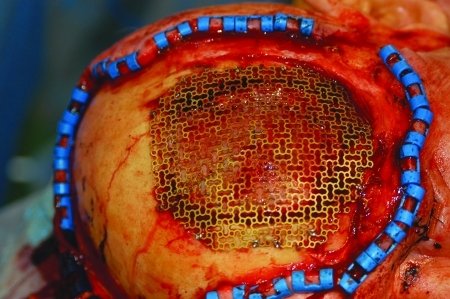
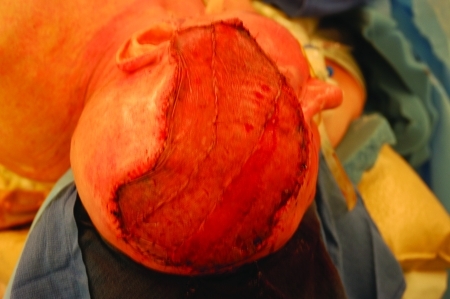
The calvarial resection is usually replaced by titanium mesh that is fixed to the bone with small titanium screws (Fig. 8). The cutaneous defect is closed with a free flap taken from the anterolateral thigh, the rectus abdominis, or the latissimus dorsi. In some instances the fat and subcutaneous tissue is so thick that the flap is denuded down to the muscle and the muscle is skin grafted (Fig. 9). With time the denervated muscle atrophies and flattens out producing a more pleasing aesthetic result (Fig. 10).
Figure 8.
Titanium mesh placed to replace resected calvarium.
Figure 9.
De-epithelialized latissimus dorsi flap used to replace scalp defect. Flap covered with split-thickness skin graft.
Figure 10.
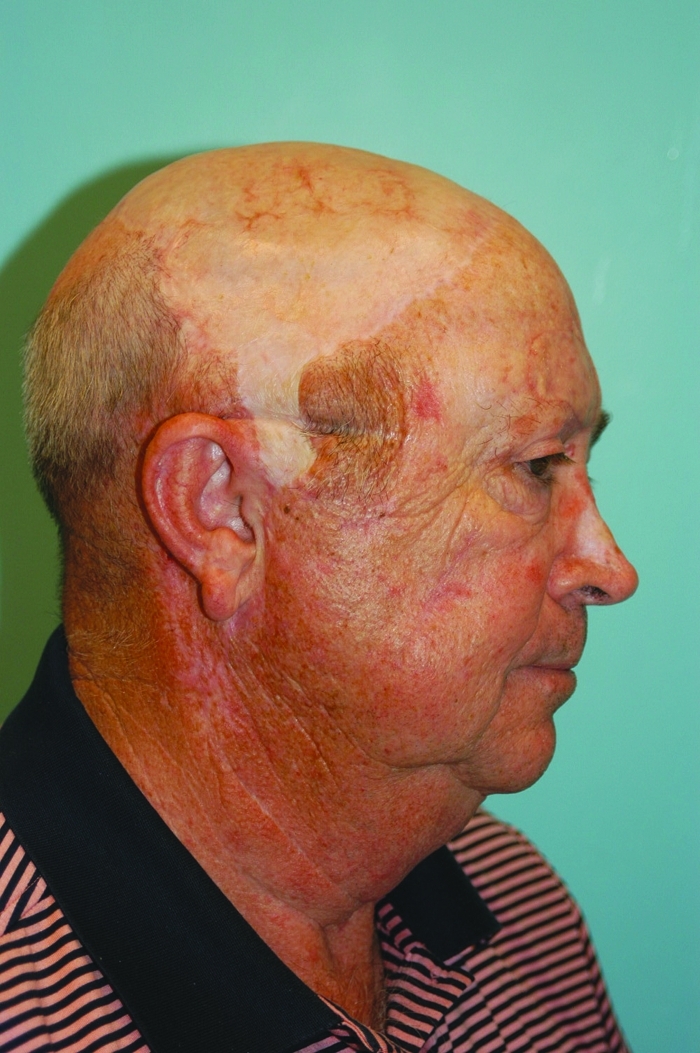
Patient 2.5 years postoperatively. Muscle flap has shrunk and is aesthetically acceptable.
RESULTS
Of the 52 patients in the series 4 elected not to have treatment at UC Davis and sought treatment elsewhere or had insufficient data to be included in the study. One of the remaining patients was lost to follow-up. The average length of follow-up period was 30.1 months with the shortest being 4 months and the longest 110 months. Only one patient was lost to follow-up. The average age of the patients was 64.7 years with the youngest aged 22 and the oldest 89. There were 12 patients who were over the age of 80.
The distribution of tumor cell types is shown in Table 2. The commonest malignancy was squamous cell carcinoma, seen in 26 of the 52 or 50%, and the second most common was basal cell carcinoma: 9 of the 52 or 17%.
Table 2.
UC Davis Experience, 1998 to 2010: 52 Cases—Cell Type New
| Basal cell carcinoma = 9 |
| Squamous cell carcinoma = 26 |
| Malignant melanoma = 6 |
| Malignant fibrous histiocytoma = 7 |
| Adenocarcinoma = 1 |
| Angiosarcoma = 2 |
| Eosinophilic granuloma = 1 |
Table 1 describes the numbers of the 48 patients who elected to be treated by surgery that fell into the three treatment groups based on the depth of invasion. There were 17 in the “superficial group,” 20 that were “intermediate,” and 11 who were classified as “deep.”
Most of the patients in the group had already multiple past excisions and many had prior radiation therapy. Table 3 lists the previous treatment for the group, and any postoperative adjuvant therapy they received after their definitive surgery at UC Davis.
Table 3.
Prior Therapy Deep Scalp Cancer
| 0 Prior Rx = 10 |
| 1 Surgical resection = 13 |
| >1 Surgical resection = 11 |
| Radiation Rx alone = 0 |
| Surgery + radiation = 7 |
| Cryotherapy = 1 |
Table 4 lists survival for patients with extensive invasion of scalp carcinoma who were followed for a minimum of 2 years or to death. There were 36 patients in this group, 21 of whom are alive without disease for a 2-year and better for a survival rate of 58.3%. As there are many elderly patients in this group it is expected that many would die of intercurrent disease. Only five died of their disease, one of which had no evidence of disease locally but died of distant metastatic disease. Nine patients (25%) died of other causes; the singular case of local control but dead of distant metastases has already been mentioned. Of the remaining 8, 4 were free of disease and 2 had persistent tumor. Local control rate was then 77.8%.
Table 4.
Survival Outcome in 36 Patients
| Alive NED = 21 |
| Alive with disease = 2 |
| Dead of disease = 4 |
| Dead of other causes: |
| With local disease = 2 |
| NED = 2 |
| Dead meets NED locally = 1 |
When survival was stratified as related to depth of invasion the differences did not reach statistical significance.
DISCUSSION
Moh's micrographic technique is highly successful for superficially invasive scalp tumors. Most deeper malignancies of the scalp are treated by local excision with primary closure, local flap advancement, or split-thickness skin grafting. The presence of negative resection margins ensures the best chance of the tumor ablation. However, in some instances despite the overall efficacy of the Moh's technique in some patients the tumor recurs (Fig. 2). In other patients the tumors are badly neglected for long periods of time and present in an advanced stage (Fig. 3). The subject of this communication principally deals with these advanced lesions that are often recurrent or in whom treatment has been avoided, often for many years.
There is very little in the literature discussing the management of carcinomas that arise primarily in the scalp. Aspoas et al16 presented a series of 23 cases that were described as extensive vault and skull base resections reconstructed with free flaps. There were 19 patients with tumor, 12 had craniofacial tumors, and only 7 patients had scalp cancer while the remainder had either, a glioma, trauma, infection, or radionecrosis of the calvarium. Of the seven patients with dural and bone involvement, repair was necessary in five patients. There were 4 local recurrences in the entire series of 19 tumor patients. Although an excellent algorithm for reconstruction of various forms of scalp defects, from a wide ranges of causes, was described by Leedy et al,17 virtually nothing was devoted to the management of cancer with deep invasion. Newman et al18 describe a 15-year review of 64 patients who had 74 separate procedures for various tumors. Skin cancer accounted for 50 (68.5%) of the cases. Craniotomy was performed in 24 of the procedures but no mention was made of how many had craniectomies or outer table calvarial resection. Primary closure was done in 3 procedures, skin grafts in 13, local flaps in 29, and free flaps in 28. Previous radiation and previous surgery were the two main determinants of complications.
A recent study by Iblher et al19 of 60 patients with scalp tumors gathered over a 10-year period was reported on. Basal cell carcinoma dominated their series while squamous cell carcinoma was the commonest diagnosis seen in our patients. Primary closure with primary approximation was possible in 21 (35%) of their patients but was possible in only 1 of our cases. Free flap reconstruction was performed in 16 (26.7%) of their cases and was used in our series in 12 (25%).
Unfortunately the depth of invasion was not mentioned in their series. One illustrative case showed a deep invasion type of patient who underwent craniectomy and dural resection with duraplasty and free flap reconstruction. The survival rate in the Ibhler et al's series was better than ours but was recorded only as tumor-related deaths rather than overall survival. In addition 35% of their patients had lesions small enough to be closed primarily.
A collaborative study including 84 scalp resections done at Erasmus University in Rotterdam and the University of Toronto in Canada was recently reported by van Driel et al.20 Included in their series were 29 patients with forehead defects. Full thickness cranial defects were necessary to achieve complete resection in 38 or 46% of patients and dural defects were created in 13 or 16% of patients. They created a complex but very instructive treatment algorithm for reconstruction. Much of the article was devoted to the method of reconstruction and the results thereof. Those that had intracranial invasion or melanoma had the worst survival.
Our series of patients were from retrospective analysis of data extracted from a prospectively acquired computerized database of our cases involving the calvarium with or without dural invasion. Our treatment algorithm applied to this series of cases produced satisfactory results.
CONCLUSION
Advanced scalp cancer is often considered inoperable when the dura is invaded and even more so if the underlying brain is involved. In our series of scalp cancer invading the calvarium with deep invasion our 2-year and better tumor-free survival rate of cancer, (58.3%) and local control rate of (77.8%) is a testimony to the application of the interdisciplinary principles of skull base surgery to the management of these challenging and sometimes daunting cases.
References
- Sewell D A, Lai S Y, Weber R S. Non-melanoma skin cancer of the head and neck. In: Suen J Y, Meyers J N, Hanna E, editors. Cancer of the Head and Neck. 4th ed. Philidelphia, PA: WB Saunders/Elsevier; 2003. pp. 117–121. [Google Scholar]
- Ananthaswamy H N, Pierceall W E. Molecular mechanisms of ultraviolet radiation carcinogenesis. Photochem Photobiol. 1990;52(6):1119–1136. doi: 10.1111/j.1751-1097.1990.tb08452.x. [DOI] [PubMed] [Google Scholar]
- Buzzell R A. Carcinogenesis of cutaneous malignancies. Dermatol Surg. 1996;22(3):209–215. doi: 10.1111/j.1524-4725.1996.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Buzzell R A. Effects of solar radiation on the skin. Otolaryngol Clin North Am. 1993;26(1):1–11. [PubMed] [Google Scholar]
- Fitzpatrick T B. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124(6):869–871. doi: 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]
- Coleman W P, III, Yarborough J M, Mandy S H. Dermabrasion for prophylaxis and treatment of actinic keratoses. Dermatol Surg. 1996;22(1):17–21. doi: 10.1111/j.1524-4725.1996.tb00565.x. [DOI] [PubMed] [Google Scholar]
- Kao G F, Graham J K. Premalignant cutaneous disorders of the head and neck. In: English G M, editor. Otolaryngology. Vol 5. New York, NY: Harper & Row; 1986. pp. 1–20. [Google Scholar]
- Sm R, Flynn J, DeGuzman M J, Silverman A R, Neiland M L. Pathology of selected skin lesions of the head and neck. In: Barnes L, editor. Surgical Pathology of the Head and Neck. Vol 3. 2nd ed. Vol. 26. New York: Marcel Deckker; 2001. pp. 1793–1875. [Google Scholar]
- Inagi R, Kosuge H, Nishimoto S, Yoshikawa K, Yamanishi K. Kaposi's sarcoma-associated herpesvirus (KSHV) sequences in premalignant and malignant skin tumors. Arch Virol. 1996;141(11):2217–2223. doi: 10.1007/BF01718227. [DOI] [PubMed] [Google Scholar]
- Mohs F E. Chemosurgery: microscopically controlled surgery for skin cancer—past, present and future. J Dermatol Surg Oncol. 1978;4(1):41–54. doi: 10.1111/j.1524-4725.1978.tb00379.x. [DOI] [PubMed] [Google Scholar]
- Tromovitch T A, Stegman S J. Microscopie-controlled excision of cutaneous tumors: chemosurgery, fresh tissue technique. Cancer. 1978;41(2):653–658. doi: 10.1002/1097-0142(197802)41:2<653::aid-cncr2820410232>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Orticochea M. Four flap scalp reconstruction technique. Br J Plast Surg. 1967;20(2):159–171. doi: 10.1016/s0007-1226(67)80032-8. [DOI] [PubMed] [Google Scholar]
- Donald P J. Transfacial approach. In: Donald P J, editor. Surgery of the Skull Base. Philadelphia, PA: Lippincott-Raven; 1998. pp. 165–194. [Google Scholar]
- Kinney S E. Squamous cell carcinoma of the external auditory canal. Am J Otol. 1989;10(2):111–116. [PubMed] [Google Scholar]
- Ananthaswamy H N, Pierceall W E. Molecular mechanisms of ultraviolet radiation carcinogenesis. Photochem Photobiol. 1990;52(6):1119–1136. doi: 10.1111/j.1751-1097.1990.tb08452.x. [DOI] [PubMed] [Google Scholar]
- Aspoas A R, Wilson G R, McLean N R, Mendelow A D, Crawford P J. Microvascular reconstruction of complex craniofacial defects. Ann R Coll Surg Engl. 1997;79(4):278–283. [PMC free article] [PubMed] [Google Scholar]
- Leedy J E, Janis J E, Rohrich R J. Reconstruction of acquired scalp defects: an algorithmic approach. Plast Reconstr Surg. 2005;116(4):54e–72e. doi: 10.1097/01.prs.0000179188.25019.6c. [DOI] [PubMed] [Google Scholar]
- Newman M I, Hanasono M M, Disa J J, Cordeiro P G, Mehrara B J. Scalp reconstruction: a 15-year experience. Ann Plast Surg. 2004;52(5):501–506. discussion 506. doi: 10.1097/01.sap.0000123346.58418.e6. [DOI] [PubMed] [Google Scholar]
- Iblher N, Ziegler M C, Penna V, Eisenhardt S U, Stark G B, Bannasch H. An algorithm for oncologic scalp reconstruction. Plast Reconstr Surg. 2010;126(2):450–459. doi: 10.1097/PRS.0b013e3181e09515. [DOI] [PubMed] [Google Scholar]
- Driel A A van, Mureau M AM, Goldstein D P, et al. Aesthetic and oncologic outcome after microsurgical reconstruction of complex scalp and forehead defects after malignant tumor resection: an algorithm for treatment. Plast Reconstr Surg. 2010;126(2):460–470. doi: 10.1097/PRS.0b013e3181de2260. [DOI] [PubMed] [Google Scholar]