ABSTRACT
Gram-negative bacteria naturally produce outer membrane vesicles (OMVs) that arise through bulging and pinching off of the outer membrane. OMVs have several biological functions for bacteria, most notably as trafficking vehicles for toxins, antimicrobials, and signaling molecules. While their biological roles are now appreciated, the mechanism of OMV formation has not been fully elucidated. We recently demonstrated that the signaling molecule 2-heptyl-3-hydroxy-4-quinolone (PQS) is required for OMV biogenesis in P. aeruginosa. We hypothesized that PQS stimulates OMV formation through direct interaction with the outer leaflet of the outer membrane. To test this hypothesis, we employed a red blood cell (RBC) model that has been used extensively to study small-molecule–membrane interactions. Our results revealed that addition of PQS to RBCs induced membrane curvature, resulting in the formation of membrane spicules (spikes), consistent with small molecules that are inserted stably into the outer leaflet of the membrane. Radiotracer experiments demonstrated that sufficient PQS was inserted into the membrane to account for this curvature and that curvature induction was specific to PQS structure. These data suggest that a low rate of interleaflet flip-flop forces PQS to accumulate in and expand the outer leaflet relative to the inner leaflet, thus inducing membrane curvature. In support of PQS-mediated outer leaflet expansion, the PQS effect was antagonized by chlorpromazine, a molecule known to be preferentially inserted into the inner leaflet. Based on these data, we propose a bilayer-couple model to describe P. aeruginosa OMV biogenesis and suggest that this is a general mechanism for bacterial OMV formation.
IMPORTANCE
Despite the ubiquity and importance of outer membrane vesicle (OMV) production in Gram-negative bacteria, the molecular details of OMV biogenesis are not fully understood. Early experiments showed that 2-heptyl-3-hydroxy-4-quinolone (PQS) induces OMV formation through physical interaction with the membrane but did not elucidate the mechanism. The present study demonstrates that PQS specifically and reversibly promotes blebbing of model membranes dependent upon the same properties that are required for OMV formation in P. aeruginosa. These results are consistent with a mechanism where expansion of the outer leaflet relative to the inner leaflet induces localized membrane curvature. This “bilayer-couple” model can account for OMV formation under all conditions and is easily generalized to other Gram-negative bacteria. The model therefore raises the possibility of a universal paradigm for vesicle production in prokaryotes with features strikingly different from what is known in eukaryotes.
Introduction
Bacteria utilize complex trafficking schemes to transport toxins and signals to target cells. An understudied yet important trafficking mechanism involves the use of outer membrane vesicles (OMVs) by Gram-negative bacteria. OMVs are spherical, bilayered vesicles derived from the outer membrane that range in size from 20 to 500 nm (1–9). Similar to the outer membrane, OMVs possess an outer leaflet of lipopolysaccharide (LPS) and an inner leaflet of phospholipid (1–9). OMVs also contain outer membrane proteins and entrap periplasmic components, presumably in the lumen, as they are released (10–12). OMVs have been found associated with Gram-negative bacteria growing planktonically and in surface-attached biofilm communities, in solid or liquid media, and in natural environments (2, 13–15). Once thought to be artifacts of cell division, they are now known to have several important biological functions, including delivery of toxins to prokaryotic and eukaryotic cells (16–18), modulation of the immune system (13), trafficking of signaling molecules between bacterial cells (18), and serving as a potential structural component of surface-associated biofilm communities (14).
Despite the known roles of OMVs in many important biological processes, the molecular details of OMV biogenesis have not been fully elucidated. A number of models have been proposed based largely on either a loss of lipoprotein connections between the bacterial outer membrane and the underlying peptidoglycan layer (19–23) or accumulation of misfolded proteins in the periplasmic space (24–26). These models are not mutually exclusive and, indeed, have many features in common. However, since enhancement of OMV formation by these routes requires genetic manipulation to delete specific lipoproteins or induce a membrane stress response, these models fail to explain how OMVs are generated during “normal” growth in wild-type bacteria.
The opportunistic pathogen Pseudomonas aeruginosa represents a (so far) unique system in that it secretes an endogenously produced small molecule, the Pseudomonas quinolone signal 2-heptyl-3-hydroxy-4-quinolone (PQS) (Fig. 1), which is necessary and sufficient for OMV formation (18, 27). While initially described as a bacterial signal important for controlling group behaviors (28), PQS has subsequently been shown to be a multifunctional molecule (29). Interestingly, elimination of the PQS receptor did not alter PQS-mediated OMV stimulation in P. aeruginosa (18), leading us to predict that PQS induces OMV biogenesis by a physical process that is specific to its structure rather than through signaling (18, 30, 31). Because of their roles in cell-cell communication and pathogenesis, elucidating the molecular mechanism of OMV biogenesis will provide new insight into P. aeruginosa signaling and disease and potentially provide a novel target for antimicrobial development.
FIG 1 .
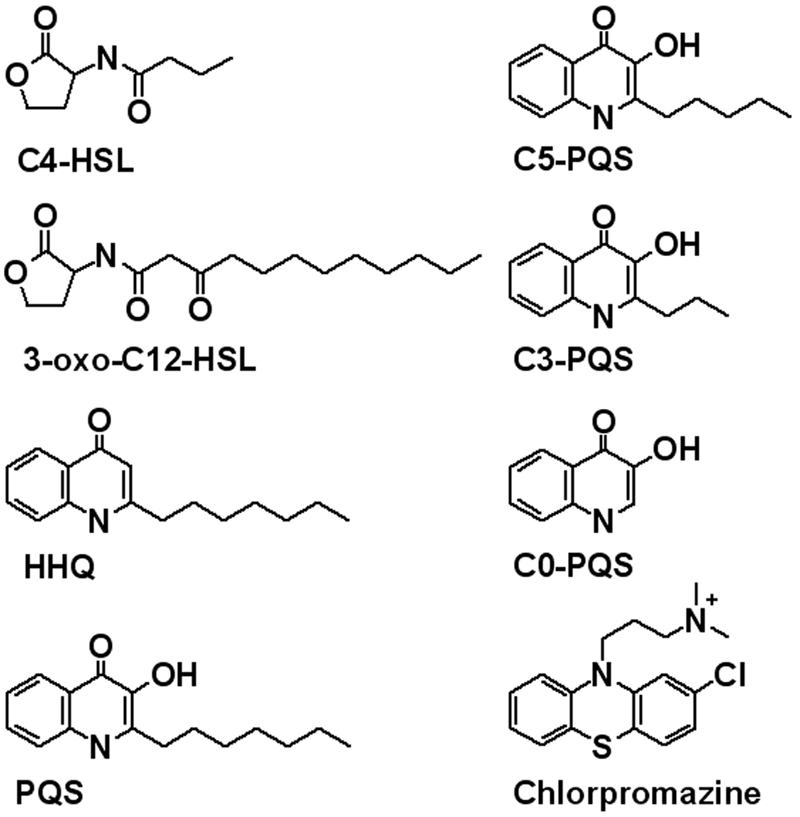
Structures of the compounds used in this study.
The introduction of curvature into the outer membrane is necessarily one of the first prerequisites of OMV biogenesis. Because of its hydrophobic nature (Table 1) and strong interactions with LPS (30), we hypothesized that PQS contributes to OMV biogenesis through the induction of membrane curvature. To test this hypothesis, we sought a model system where the contribution of PQS to membrane curvature could be tested in vitro; however, reconstitution of the asymmetric lipid bilayer of the bacterial outer membrane into liposomes or lipid films has not been achieved. Fortuitously, the question of curvature induction by exogenously added small molecules has been thoroughly investigated using red blood cells (RBCs) (32–35). Lacking a nucleus, RBCs essentially serve as complex giant unilamellar vesicles that are unlikely to have PQS-specific receptors and are incapable of altering gene expression in response to exogenous compounds. Thus, they have the potential to serve as a largely inert system to test the propensity of PQS to induce membrane curvature. What is perhaps most appealing about RBCs, as opposed to liposomes, is that they undergo obvious and well-documented shape changes in response to membrane-intercalating agents (32–35).
TABLE 1 .
Sizes and hydrophobicities of the compounds used in this study
| Compound | Molecular mass (g/mol) | Avg clogP a value ± SD |
|---|---|---|
| Chlorpromazine | 318.90 | 4.88 ± 0.48b |
| HHQc | 243.38 | 4.80 ± 0.31 |
| PQS | 259.38 | 4.34 ± 0.60 |
| C5-PQS | 231.32 | 3.28 ± 0.63 |
| 3-Oxo-C12-HSL | 297.44 | 3.21 ± 0.82 |
| C3-PQS | 203.26 | 2.30 ± 0.48 |
| C0-PQS | 161.17 | 0.93 ± 0.64 |
| C4-HSL | 171.22 | 0.29 ± 0.41 |
clogP (calculated log octanol-water partition coefficient) values were estimated using ALOGPS 2.1 (http://www.vcclab.org/lab/alogps/start.html)(53).
Experimentally determined to be 5.41 (PhysProp database entry for CAS 50-53-3).
HHQ, 2-heptyl-4-quinolone.
In 1974, Sheetz and Singer proposed that the response of a lipid bilayer to amphiphilic small molecules was analogous to the response of a bimetallic thermocouple to heat (35). That is, if the amphiphilic molecule were to concentrate in one membrane leaflet, whether by specific attraction to that leaflet or slow flip-flop, that leaflet would expand relative to the other and cause the membrane as a whole to curve. This idea was dubbed the bilayer-couple hypothesis (35), and Sheetz and Singer adopted the RBC as their model system. Many experiments have shown that amphiphilic molecules with diverse structures can induce the crenation (outward bulging of the membrane, spicule formation) or cup formation (inward bulging of the membrane) predicted by this hypothesis (34–38), and examination of the leaflet distribution of individual small molecules has lent additional support (33). More recently, the mechanics of RBC shape change were mathematically modeled and a single parameter, the ratio of leaflet areas, could describe the entire shape change phenomenon (39). In the work described here, we used the bilayer-couple RBC model to show that PQS specifically induces curvature in RBC membranes in a concentration-dependent manner. Other predictions of the bilayer-couple hypothesis were also satisfied, and we propose that asymmetric expansion of the outer leaflet of the outer membrane be incorporated into a mechanistic model of P. aeruginosa OMV formation.
RESULTS
PQS induces RBC hemolysis.
RBCs have been used for decades to study the dissolution of small molecules into biological membranes, specifically focusing on how these molecules impact RBC shape (34–38). A key advantage of RBCs over liposomes or lipid films is that, analogous to the bacterial outer membrane, the cytoplasmic membrane is linked to an underlying cytoskeleton. This attachment provides stability to resulting shape changes and prevents vesiculation, allowing membrane curvature caused by small molecules to be stable and observed in real-time (39). Based on our recent hypothesis that PQS is confined to the outer leaflet of the bacterial outer membrane (30, 40), we sought to examine the impact of PQS on RBC shape. Previous studies demonstrated that, above a certain threshold, insertion of small molecules into the outer leaflet of the RBC membrane resulted in cell lysis (35). To test whether addition of PQS to RBCs also induces lysis, we exposed RBCs to increasing concentrations of the molecule, from the low end of the physiological range (5 µM) to beyond the physiological range (80 µM). PQS induced a linear increase in hemoglobin release (a measure of hemolysis) beginning at ~10 µM (Fig. 2). At 80 µM, the extent of hemolysis was ~70% of that of the hypotonic-lysis positive control. Thus, RBCs were able to tolerate low concentrations of PQS, but as concentrations reached common physiological levels (25 to 50 µM), many cells were lysed. This response is consistent with the proposal that PQS dissolves into the membrane, where it causes morphological changes that eventually lead to hemolysis. For subsequent experiments, PQS was used at 5 µM (unless otherwise noted) so that morphological effects could be observed without risk of hemolysis.
FIG 2 .
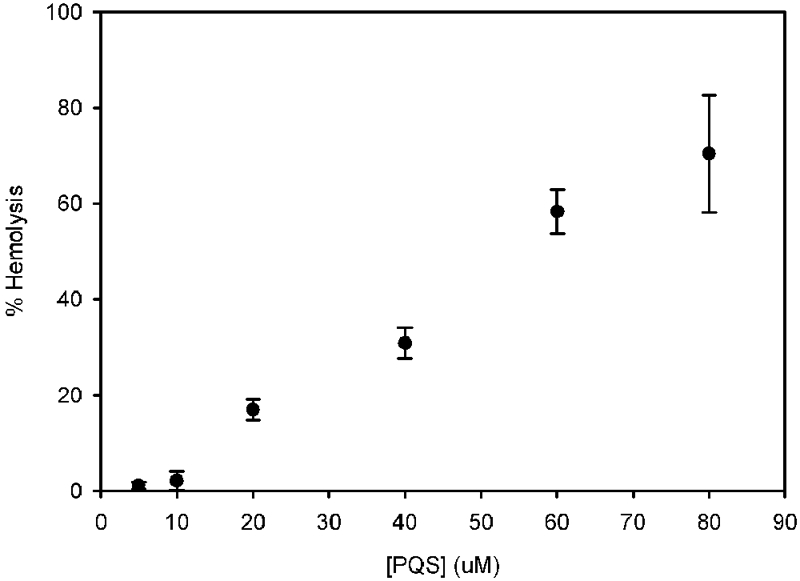
PQS induces RBC hemolysis. Washed RBCs were exposed to various amounts of PQS for 30 min at room temperature. Intact cells were removed by centrifugation, and hemoglobin release was assessed by measuring the absorbance of the supernatant at 543 nm. Results are presented as percent hemolysis relative to that of a hypotonic-lysis control where RBCs were lysed by resuspension in 10 mM Tris, pH 7.5. Each point represents the average of three independent measurements ± the standard error. HHQ, 2-heptyl-4-quinolone.
PQS induces curvature in RBC membranes.
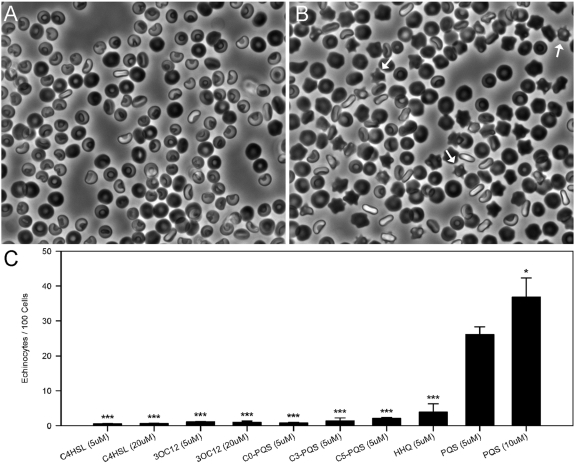
Since PQS induces RBC lysis in a manner similar to that of many other curvature-inducing small molecules, we hypothesized that PQS would induce membrane curvature at low concentrations. To test this hypothesis, we adapted an existing RBC system to allow membrane curvature to be observed in real time in unfixed cells. By allowing RBCs to interact with PQS and then settle onto the bottom of a chamber slide, the “glass effect” (41) and any artifacts potentially introduced by chemical fixation were avoided. In support of the bilayer-couple hypothesis, low concentrations of PQS induced RBC crenation to form echinocytes (RBCs with outward membrane protrusions) (Fig. 3A to C). A higher proportion of RBCs were crenate when treated with 10 µM than when treated with 5 µM PQS (Fig. 3C), suggesting a concentration-dependent response. Though significantly higher (P < 0.05), the percentage of RBCs affected at 10 µM PQS was not double that seen at 5 µM. This may be due to bias introduced by a small amount of hemolysis (Fig. 2) since crenation presumably precedes lysis.
FIG 3 .
PQS induces RBC membrane curvature. Compounds were added to washed RBCs and observed using light microscopy after 30 min. (A) RBCs treated with DMSO only. (B) RBCs treated with 5 µM PQS; white arrows point to echinocytes (spicule-containing cells). (C) RBCs were exposed to a series of compounds to assess the impact of chemical structure on the ability to promote membrane curvature. The bars represent the average number of echinocytes per 100 cells. Averages were calculated from at least three independent measurements (≥6,800 total cells). Error bars show the standard errors of the means. Asterisks represent statistically significant differences from PQS (5 µM) using a one-tailed t test. *, P < 0.05; ***, P ≤ 0.0001. HHQ, 2-heptyl-4-quinolone.
Quantification of PQS associated with RBCs.
In order to promote curvature, the bilayer-couple model demands that a sufficient amount of PQS intercalate into the outer leaflet of the RBC membrane. To examine PQS incorporation into RBCs, we incubated 5 µM [3H]PQS with RBCs for 30 min at room temperature. RBCs were then separated from the supernatant by centrifugation, washed, and analyzed by scintillation counting. Results from these experiments revealed that, if evenly distributed, each RBC contained 3.2 × 106 ± 0.4 × 106 PQS molecules (average ± standard error).
To determine if the insertion of this number of PQS molecules is sufficient to induce the RBC membrane curvature observed, we used the Avogadro chemical analysis program (version 1.0.3, http://avogadro.openmolecules.net/) to estimate the Van der Waals cross-sectional area of PQS. Assuming a perfectly planar conformation, adding the distance between the C-4 oxygen and N-1 hydrogen of PQS to the Van der Waals radii of oxygen and hydrogen gave a “width” of 0.78 nm. This value multiplied by the “thickness” of PQS, which is equal the diameter of carbon (0.34 nm), gave a cross-sectional area of 0.26 nm2/molecule. The conformation PQS adopts in phospholipid or LPS membranes is not known, but this value represents the minimum possible PQS surface area. Using similar methodology, a maximum estimate for the extended molecule was calculated to be 1.4 nm2/molecule. Based on these values, incorporation of 3.2 × 106 PQS molecules into a single RBC would cause a 0.6 to 3.2% increase in outer leaflet area relative to the inner leaflet area. This range agrees well with data observed for the curvature-inducing molecule phosphatidylcholine (1.7%) (42) and is within the expansion range (0.4% to 0.9%) predicted theoretically to be necessary for curvature induction (39). It is noteworthy that only a portion of the RBC population developed spicules in our experiments. This suggests that exogenous PQS was not delivered equally to each cell, perhaps as a result of incorporation of PQS micelles rather than individual molecules; thus, some RBCs may have significantly higher levels of incorporated PQS. Regardless, we observed sufficient association of PQS with RBCs to fall within both theoretical (39) and empirical (42) expectations of what would be necessary to induce spicule formation by expansion of the membrane’s outer leaflet.
Curvature induction is specific to PQS structure.
In order to better understand the nature of the PQS effect, we performed structure-activity relationship experiments using PQS analogs (Fig. 1; Table 1). There was a striking loss of curvature-inducing potential when either the PQS alkyl chain was shortened or the 3-hydroxyl group was eliminated (Fig. 3C). As nonquinolone controls for these experiments, we also tested the ability of the two secreted P. aeruginosa acyl-homoserine lactone signals (Fig. 1) to alter RBC shape. Butyryl-homoserine lactone (C4-HSL) and 3-oxo-dodecanoyl-homoserine lactone (3-oxo-C12-HSL) have not been observed to promote OMV formation (18), despite the fact that insertion of 3-oxo-C12-HSL into phospholipid membranes has been demonstrated (43). Even at four times the concentration, neither homoserine lactone molecule induced curvature in RBC membranes (Fig. 3C). Attempts to analyze the more hydrophobic acyl-homoserine lactone signals produced by other bacteria were hampered by their inability to dissolve in dimethyl sulfoxide (DMSO), the only solvent tested that was well tolerated by RBCs.
Curvature induction can be predictably reversed.
Since the bilayer-couple model predicts that spicule formation is the result of asymmetric expansion of the outer leaflet relative to the inner one, this effect should be directly antagonized by a small molecule that can preferentially expand the inner leaflet. This antagonism has been demonstrated using mixtures of oleate and chlorpromazine, molecules well known to induce RBC crenation and cup formation, respectively (34). To test whether the effect of PQS is consistent with this prediction, we exposed RBCs to both PQS and chlorpromazine. Titration of increasing amounts of chlorpromazine into samples of PQS-treated RBCs resulted in a concentration-dependent antagonistic effect (Fig. 4), with crenation completely abolished when PQS is present at a ratio of 1:1 with chlorpromazine. Thus, the bilayer-couple model describes a consistent, specific effect of PQS that can be predictably antagonized by a molecule known to produce the opposite effect.
FIG 4 .
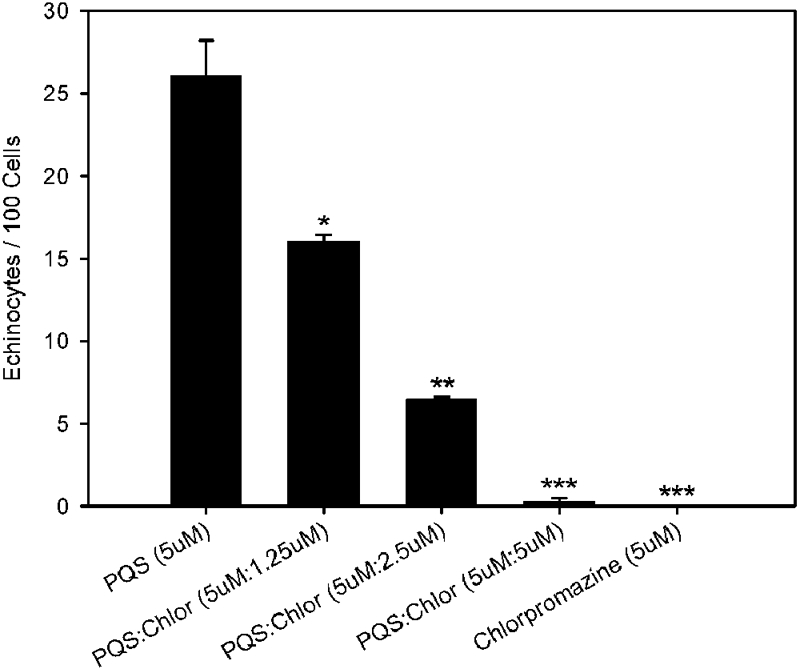
The PQS effect is antagonized by chlorpromazine. Washed RBCs were exposed to PQS, both PQS and chlorpromazine, or chlorpromazine alone. The bars represent the average numbers of echinocytes per 100 cells. Averages were calculated from at least three independent measurements (≥6,800 total cells). Error bars show the standard errors. Asterisks represent statistically significant differences from PQS (5 µM) using a one-tailed t test. *, P < 0.05; **, P < 0.0002; ***, P < 0.0001. Chlor, chlorpromazine.
DISCUSSION
OMVs are well recognized trafficking vehicles for several biologically active molecules, including toxins, antimicrobials, and quorum signals. Although enhanced OMV formation occurs in response to a membrane stress response in Escherichia coli (24), most Gram-negative bacteria naturally produce OMVs while growing under perceived optimal, stress-free conditions (44). The goal of this study was to develop a robust model of OMV biogenesis during “normal” growth. Previous studies in our laboratory and others were instrumental in developing this model, including those showing that PQS, but not PQS signaling, is required for P. aeruginosa OMV formation (25, 30, 31, 40, 45); exogenous addition of PQS stimulates OMV formation in other Gram-negative bacteria (30); PQS accumulates in OMVs, suggesting that PQS may nucleate in the membrane (18, 40); PQS possesses higher affinity for LPS than phospholipids, likely due to specific interactions with lipid A (30); and the addition of PQS to LPS aggregates induces the formation of OMV-sized LPS liposomes (30). Based on these observations and the data presented in this study, we propose a model where PQS induces OMV biogenesis by accumulating in the LPS-rich outer leaflet of the outer membrane, resulting in a bilayer-couple effect that induces membrane curvature, ultimately leading to pinching off of the OMV due to physical forces (Fig. 5). We submit that this is the dominant mechanism governing OMV formation in P. aeruginosa under standard in vitro growth conditions and that the secretion of membrane-active molecules could be a general mechanism of OMV formation in many Gram-negative bacteria.
FIG 5 .
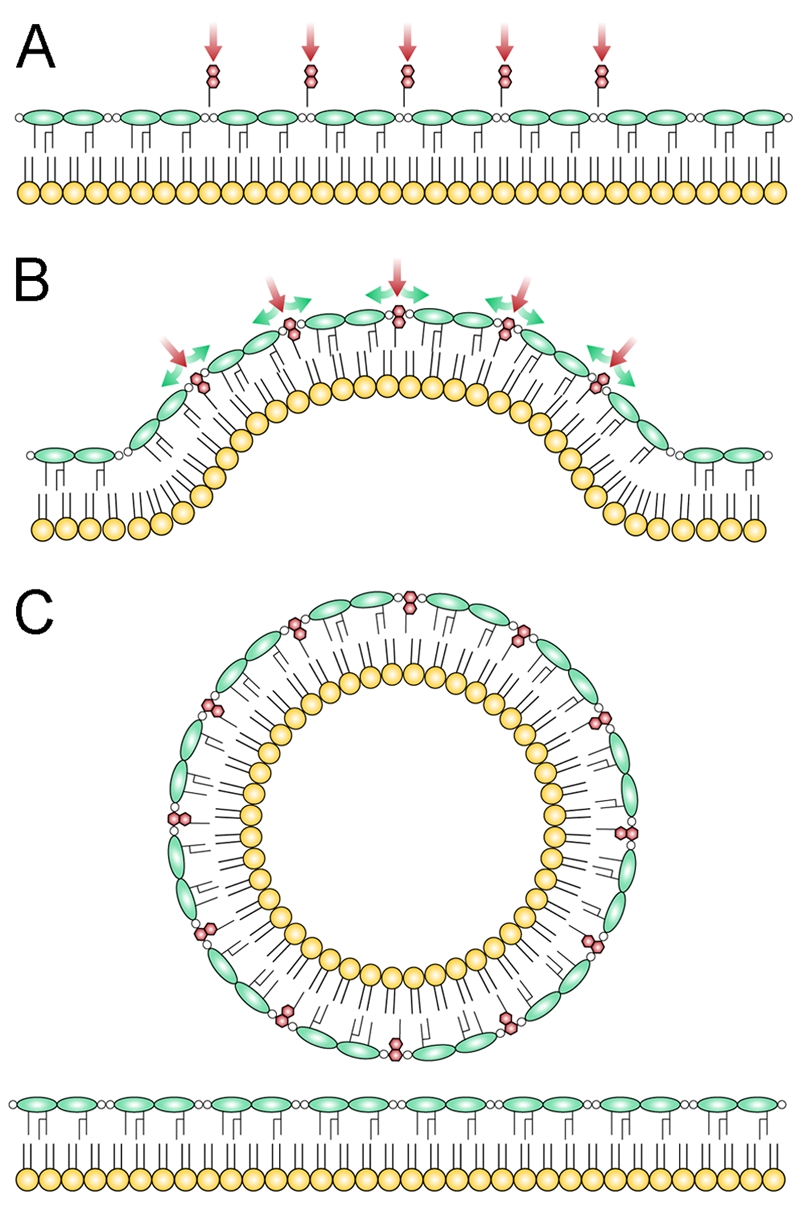
Bilayer-couple model of OMV formation in P. aeruginosa. The model proposes that PQS induces OMV formation through a mechanism of asymmetric expansion of the outer leaflet of the outer membrane. (A) Whether PQS is added exogenously or exported from the producing cell, the hydrophobic nature of PQS causes it to associate with LPS, where it makes specific contacts with the 4′-phosphate and acyl chains of lipid A. (B) These specific interactions contribute to a low rate of flip-flop between leaflets, causing PQS to expand the outer leaflet relative to the inner leaflet. When the ratio of the leaflet areas becomes sufficiently large, the membrane as a whole is forced to buckle in response to this asymmetry. (C) With continuing insertion of PQS, curvature increases until OMVs, which can accommodate a large ratio of outer to inner leaflet area, bud from the surface. Red, PQS; green, LPS; yellow, phospholipids. Polysaccharides have been omitted from LPS for clarity.
The bilayer-couple model makes a number of testable predictions that we addressed in this study. It has long been appreciated that intercalation of membrane-active small molecules eventually leads to RBC hemolysis (reviewed in reference 46), and we showed that increasing concentrations of PQS positively correlate with hemolysis (Fig. 2). Interestingly, PQS caused appreciable hemolysis at concentrations well within the physiological range produced by planktonically grown P. aeruginosa (47). Though PQS was not previously thought to be a virulence factor itself (28), this finding suggests that PQS may contribute to OMV cytotoxic effects. Low concentrations of PQS also caused RBCs to become crenate—the expected response to a molecule that accumulates in the outer leaflet (Fig. 3). Unlike with LPS (30), PQS is not known to interact specifically with RBC phospholipids. Rather, an inherently low rate of PQS flip-flop between leaflets is likely responsible for spicule formation in RBCs. We predict that the inability to rapidly equilibrate between leaflets is more pronounced in the bacterial outer membrane due to strong interactions with LPS in the outer leaflet (30). Interestingly, the presence of both the alkyl chain and 3-hydroxyl components of PQS was required for RBC curvature induction (Fig. 3C), in agreement with the importance of these substituents for OMV formation (30). These results highlight a structure-specific response of biological membranes to PQS and suggest that the RBC system could be useful in identifying OMV-promoting small molecules from other organisms.
The strongest test of the bilayer-couple model was provided by experiments combining PQS with chlorpromazine. Chlorpromazine induces RBC invagination (cup formation) through accumulation in the inner leaflet, where it makes favorable ionic interactions with phospholipids residing there (34). If PQS acts to induce RBC spicule formation through expansion of the outer leaflet of the membrane, it is logical that this effect would be counteracted by a molecule that expands the inner leaflet. We showed that treatment of RBCs with PQS and chlorpromazine together resulted in elimination of the PQS effect (Fig. 4). At a constant concentration of PQS, this antagonism was directly proportional to the concentration of chlorpromazine, with full antagonism occurring at a 1:1 ratio of the compounds. Under these conditions, the RBCs were indistinguishable from cells that received no treatment. These experiments demonstrated that the theoretical mechanisms underpinning the bilayer-couple model successfully predicted not only the response of RBCs to PQS but also conditions under which the PQS effect was negated. Thus, the model is robust and can be used to accurately describe natural phenomena.
Proposing a bilayer-couple mechanism for P. aeruginosa OMV biogenesis requires consideration of which membrane leaflet an amphiphilic compound has access to. Clearly, when PQS is added exogenously to either RBCs or bacteria, as was the case in our in vitro experiments, it has access to the outer leaflet of the membrane. But is this the case for naturally produced PQS biosynthesized in the cytoplasm (27)? Because of its strongly hydrophobic character (Table 1), PQS is unlikely to freely diffuse out of the cell. While the mechanism of PQS export is not fully understood, it has been proposed that quinolones such as PQS are substrates for RND family efflux pumps (48–50). Natural export of PQS via active efflux would deliver the molecule directly to the outer surface of the bacterium, where it could be intercalated into the outer leaflet of the outer membrane. In this way, we expect that naturally exported PQS would act in cis on the producing cell to induce OMV formation for cell-to-cell trafficking. The fact that exogenously added PQS induces OMV formation in P. aeruginosa and other organisms strongly suggests that the outer leaflet is the target location for PQS intercalation, and the strong affinity of PQS for LPS further strengthens this claim (30).
Vesicle biogenesis is a relatively new area of study in bacteria but is well characterized in eukaryotes. However, eukaryotic vesicle production is typically mediated by protein factors that introduce curvature into the membrane (51). The proposed small-molecule-based model therefore highlights a fascinating divergence between prokaryotic and eukaryotic systems. In addition to this, the model is easily generalized, requiring only that bacteria secrete a membrane-active small molecule that accumulates in the outer leaflet of the outer membrane. Given that numerous Gram-negative bacteria produce OMVs and that microbial secreted metabolites comprise a vast chemical space, it is probable that such a bilayer-couple mechanism is widespread. The fact that PQS was the first endogenous OMV-stimulating molecule discovered likely owes more to its dual role in quorum sensing than any mechanistic uniqueness.
In sum, we propose a new model of OMV biogenesis in P. aeruginosa under normal laboratory growth conditions (Fig. 5). We predict that accumulation of PQS in the outer leaflet induces mechanical stress in the membrane that is alleviated by the protrusion of outward-facing blebs and the eventual formation of OMVs, which accommodate the increased ratio of outer to inner leaflet areas. This model unifies a number of observations regarding OMV biogenesis and provides unique insight into an understudied trafficking mechanism in prokaryotes.
MATERIALS AND METHODS
Materials.
PQS, PQS analogs, and acylhomoserine lactones were from Syntech Solutions (San Diego, CA). Compounds were dissolved in DMSO and sonicated for 10 min in a VWR Aquasonic 250T bath sonicator immediately before dilution into experimental samples. [3H]PQS (0.43 Ci/mmol) was from Amersham. Mouse RBCs were a kind gift of Andrew Ellington and were used within 1 h of harvesting. BALB/c or C57BL/6 mice were euthanized with strict adherence to institutional guidelines for minimizing distress in experimental animals. Blood was collected in a syringe via cardiac puncture, and CPD buffer (26.3 g/liter trisodium citrate dihydrate, 3.27 g/liter citric acid monohydrate, 2.22 g/liter sodium dihydrogen phosphate, 23.2 g/liter dextrose) was added to a 1:14 ratio of CPD to blood. Whole blood was washed three times by sedimentation and resuspension in TBS (10 mM Tris, 146 mM NaCl, pH 7.5) before use. Washed RBCs were diluted 400 times in TBS to give working solutions at ~0.1% hematocrit.
Hemolysis experiments.
Samples of washed RBCs (1.5 ml; ~0.1% hematocrit) were exposed to 5, 10, 20, 40, 60, or 80 µM PQS. Samples were incubated at room temperature for 30 min before intact RBCs were separated by centrifugation at 1,000 rpm for 5 min in an IEC Centra CL2 centrifuge. Supernatants were analyzed for hemoglobin release by measuring absorbance at 543 nm. The background absorbance measured in an untreated sample was subtracted from each value, and the data were normalized as a percentage of the value of the hypotonic-lysis control. For this control, washed RBCs were sedimented, resuspended in 10 mM Tris (pH 7.5), and treated as described for PQS samples.
PQS incorporation.
One-milliliter samples of washed RBCs (~0.1% hematocrit) were exposed to 5 µM [3H]PQS. Samples were incubated at room temperature for 30 min before RBCs were separated by centrifugation at 4,000 rpm for 5 min in a Spectrafuge benchtop centrifuge. The RBC pellet was washed in 1 ml TBS and resedimented before the final pellet was resuspended in 1 ml TBS and analyzed for 3H radioactivity in a Beckman Coulter LS6500 scintillation counter. Calculation of PQS insertion was based on 1010 RBCs/ml in mouse blood (52).
Echinocytosis experiments.
Five-hundred-microliter samples of washed RBCs (~0.1% hematocrit) were exposed to the experimental compounds (Fig. 2) and mixed by inversion several times. One-hundred-microliter subsamples were immediately transferred to the wells of a chambered microscope slide (Lab-Tek II Chambered 1.5 German Coverglass System) and allowed to incubate for 30 min at room temperature. Cells were then observed using a Nikon Eclipse TS100 inverted microscope with a 40× objective, and multiple fields were photographed. Micrographs were subsequently analyzed by manually counting and recording the total number of cells and the number of echinocytes observed. For chlorpromazine experiments, PQS was first added to 500 µl of washed RBCs; this was followed immediately by the addition of chlorpromazine, mixing, and transfer of the 100-µl subsample to the chamber slide.
ACKNOWLEDGMENTS
We thank Michelle Byrom for technical assistance in providing mouse blood.
This work was supported by a grant from the NIH (5R01AI075068 to M.W.). M.W. is a Burroughs Wellcome investigator in the pathogenesis of infectious disease. J.W.S. is supported by a Cystic Fibrosis Canada postdoctoral fellowship.
Footnotes
Citation Schertzer JW, Whiteley M. 2012. A bilayer-couple model of bacterial outer membrane vesicle biogenesis. mBio 3(2):e00297-11. doi:10.1128/mBio.00297-11.
REFERENCES
- 1. Beveridge TJ. 1999. Structures of gram-negative cell walls and their derived membrane vesicles. J. Bacteriol. 181:4725–4733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beveridge TJ, Makin SA, Kadurugamuwa JL, Li Z. 1997. Interactions between biofilms and the environment. FEMS Microbiol. Rev. 20:291–303 [DOI] [PubMed] [Google Scholar]
- 3. Chatterjee SN, Das J. 1967. Electron microscopic observations on the excretion of cell wall material by Vibrio cholerae. J. Gen. Microbiol. 49:1–11 [DOI] [PubMed] [Google Scholar]
- 4. Deich RA, Hoyer LC. 1982. Generation and release of DNA-binding vesicles by Haemophilus influenzae during induction and loss of competence. J. Bacteriol. 152:855–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fiocca R, et al. 1999. Release of Helicobacter pylori vacuolating cytotoxin by both a specific secretion pathway and budding of outer membrane vesicles. Uptake of released toxin and vesicles by gastric epithelium. J. Pathol. 188:220–226 [DOI] [PubMed] [Google Scholar]
- 6. Kadurugamuwa JL, Beveridge TJ. 1995. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. J. Bacteriol. 177:3998–4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kahn ME, Maul G, Goodgal SH. 1982. Possible mechanism for donor DNA binding and transport in Haemophilus. Proc. Natl. Acad. Sci. U. S. A. 79:6370–6374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li Z, Clarke AJ, Beveridge TJ. 1998. Gram-negative bacteria produce membrane vesicles which are capable of killing other bacteria. J. Bacteriol. 180:5478–5483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pettit RK, Judd RC. 1992. The interaction of naturally elaborated blebs from serum-susceptible and serum-resistant strains of Neisseria gonorrhoeae with normal human serum. Mol. Microbiol. 6:729–734 [DOI] [PubMed] [Google Scholar]
- 10. Choi DS, et al. 2011. Proteomic analysis of outer membrane vesicles derived from Pseudomonas aeruginosa. Proteomics 11:3424–3429 [DOI] [PubMed] [Google Scholar]
- 11. Ciofu O, Beveridge TJ, Kadurugamuwa J, Walther-Rasmussen J, Hoiby N. 2000. Chromosomal beta-lactamase is packaged into membrane vesicles and secreted from Pseudomonas aeruginosa. J. Antimicrob. Chemother. 45:9–13 [DOI] [PubMed] [Google Scholar]
- 12. Kesty NC, Kuehn MJ. 2004. Incorporation of heterologous outer membrane and periplasmic proteins into Escherichia coli outer membrane vesicles. J. Biol. Chem. 279:2069–2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kuehn MJ, Kesty NC. 2005. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 19:2645–2655 [DOI] [PubMed] [Google Scholar]
- 14. Schooling SR, Beveridge TJ. 2006. Membrane vesicles: an overlooked component of the matrices of biofilms. J. Bacteriol. 188:5945–5957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schooling SR, Hubley A, Beveridge TJ. 2009. Interactions of DNA with biofilm-derived membrane vesicles. J. Bacteriol. 191:4097–4102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bomberger JM, et al. 2009. Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. PLoS Pathog. 5:e1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kulp A, Kuehn MJ. 2010. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu. Rev. Microbiol. 64:163–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mashburn LM, Whiteley M. 2005. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature 437:422–425 [DOI] [PubMed] [Google Scholar]
- 19. Bernadac A, Gavioli M, Lazzaroni JC, Raina S, Lloubès R. 1998. Escherichia coli tol-pal mutants form outer membrane vesicles. J. Bacteriol. 180:4872–4878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cascales E, Bernadac A, Gavioli M, Lazzaroni JC, Lloubes R. 2002. Pal lipoprotein of Escherichia coli plays a major role in outer membrane integrity. J. Bacteriol. 184:754–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Deatherage BL, et al. 2009. Biogenesis of bacterial membrane vesicles. Mol. Microbiol. 72:1395–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Song T, et al. 2008. A new Vibrio cholerae sRNA modulates colonization and affects release of outer membrane vesicles. Mol. Microbiol. 70:100–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wensink J, Witholt B. 1981. Outer-membrane vesicles released by normally growing Escherichia coli contain very little lipoprotein. Eur. J. Biochem. 116:331–335 [DOI] [PubMed] [Google Scholar]
- 24. McBroom AJ, Kuehn MJ. 2007. Release of outer membrane vesicles by gram-negative bacteria is a novel envelope stress response. Mol. Microbiol. 63:545–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tashiro Y, et al. 2009. Outer membrane machinery and alginate synthesis regulators control membrane vesicle production in Pseudomonas aeruginosa. J. Bacteriol. 191:7509–7519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhou L, Srisatjaluk R, Justus DE, Doyle RJ. 1998. On the origin of membrane vesicles in Gram-negative bacteria. FEMS Microbiol. Lett. 163:223–228 [DOI] [PubMed] [Google Scholar]
- 27. Schertzer JW, Brown SA, Whiteley M. 2010. Oxygen levels rapidly modulate Pseudomonas aeruginosa social behaviours via substrate limitation of PqsH. Mol. Microbiol. 77:1527–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pesci EC, et al. 1999. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U. S. A. 96:11229–11234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schertzer JW, Boulette ML, Whiteley M. 2009. More than a signal: non-signaling properties of quorum sensing molecules. Trends Microbiol. 17:189–195 [DOI] [PubMed] [Google Scholar]
- 30. Mashburn-Warren L, et al. 2008. Interaction of quorum signals with outer membrane lipids: insights into prokaryotic membrane vesicle formation. Mol. Microbiol. 69:491–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mashburn-Warren LM, Whiteley M. 2006. Special delivery: vesicle trafficking in prokaryotes. Mol. Microbiol. 61:839–846 [DOI] [PubMed] [Google Scholar]
- 32. Iglic A, Kralj-Iglic V, Hägerstrand H. 1998. Amphiphile induced echinocyte-spheroechinocyte transformation of red blood cell shape. Eur. Biophys. J. 27:335–339 [DOI] [PubMed] [Google Scholar]
- 33. Matayoshi ED. 1980. Distribution of shape-changing compounds across the red cell membrane. Biochemistry 19:3414–3422 [DOI] [PubMed] [Google Scholar]
- 34. Sheetz MP, Painter RG, Singer SJ. 1976. Biological membranes as bilayer couples. III. Compensatory shape changes induced in membranes. J. Cell Biol. 70:193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sheetz MP, Singer SJ. 1974. Biological membranes as bilayer couples. A molecular mechanism of drug-erythrocyte interactions. Proc. Natl. Acad. Sci. U. S. A. 71:4457–4461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chabanel A, et al. 1983. Influence of cholesterol content on red cell membrane viscoelasticity and fluidity. Biophys. J. 44:171–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Deuticke B. 1968. Transformation and restoration of biconcave shape of human erythrocytes induced by amphiphilic agents and changes of ionic environment. Biochim. Biophys. Acta 163:494–500 [DOI] [PubMed] [Google Scholar]
- 38. Warren JR, Harris AS, Wallas CH. 1983. Transformation of human erythrocyte shape by endotoxic lipopolysaccharide. Infect. Immun. 39:431–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lim HWG, Wortis M, Mukhopadhyay R. 2002. Stomatocyte-discocyte-echinocyte sequence of the human red blood cell: evidence for the bilayer-couple hypothesis from membrane mechanics. Proc. Natl. Acad. Sci. U. S. A. 99:16766–16769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mashburn-Warren L, Howe J, Brandenburg K, Whiteley M. 2009. Structural requirements of the Pseudomonas quinolone signal for membrane vesicle stimulation. J. Bacteriol. 191:3411–3414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Eriksson LE. 1990. On the shape of human red blood cells interacting with flat artificial surfaces—the “glass effect”. Biochim. Biophys. Acta 1036:193–201 [DOI] [PubMed] [Google Scholar]
- 42. Ferrell JE, Jr, Lee KJ, Huestis WH. 1985. Membrane bilayer balance and erythrocyte shape: a quantitative assessment. Biochemistry 24:2849–2857 [DOI] [PubMed] [Google Scholar]
- 43. Davis BM, Jensen R, Williams P, O’Shea P. 2010. The interaction of N-acylhomoserine lactone quorum sensing signaling molecules with biological membranes: implications for inter-kingdom signaling. PLoS One 5:e13522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kadurugamuwa JL, Beveridge TJ. 1997. Natural release of virulence factors in membrane vesicles by Pseudomonas aeruginosa and the effect of aminoglycoside antibiotics on their release. J. Antimicrob. Chemother. 40:615–621 [DOI] [PubMed] [Google Scholar]
- 45. Hodgkinson J, Bowden SD, Galloway WR, Spring DR, Welch M. Structure-activity analysis of the Pseudomonas quinolone signal molecule. J. Bacteriol. 192:3833–3837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Seeman P. 1972. The membrane actions of anesthetics and tranquilizers. Pharmacol. Rev. 24:583–655 [PubMed] [Google Scholar]
- 47. Déziel E, et al. 2004. Analysis of Pseudomonas aeruginosa 4-hydroxy-2-alkylquinolines (HAQs) reveals a role for 4-hydroxy-2-heptylquinoline in cell-to-cell communication. Proc. Natl. Acad. Sci. U. S. A. 101:1339–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Aendekerk S, et al. 2005. The MexGHI-OpmD multidrug efflux pump controls growth, antibiotic susceptibility and virulence in Pseudomonas aeruginosa via 4-quinolone-dependent cell-to-cell communication. Microbiology 151:1113–1125 [DOI] [PubMed] [Google Scholar]
- 49. Köhler T, van Delden C, Curty LK, Hamzehpour MM, Pechere JC. 2001. Overexpression of the MexEF-OprN multidrug efflux system affects cell-to-cell signaling in Pseudomonas aeruginosa. J. Bacteriol. 183:5213–5222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lamarche MG, Déziel E. 2011. MexEF-OprN efflux pump exports the pseudomonas quinolone signal (PQS) precursor HHQ (4-hydroxy-2-heptylquinoline). PLoS One 6:e24310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schekman R, Orci L. 1996. Coat proteins and vesicle budding. Science 271:1526–1533 [DOI] [PubMed] [Google Scholar]
- 52. Russell ES, Neufeld EF, Higgins CT. 1951. Comparison of normal blood picture of young adults from 18 inbred strains of mice. Proc. Soc. Exp. Biol. Med. 78:761–766 [DOI] [PubMed] [Google Scholar]
- 53. Tetko IV, et al. 2005. Virtual computational chemistry laboratory-design and description. J. Comput. Aid. Mol. Des. 19:453–463 [DOI] [PubMed] [Google Scholar]