Figure 3.
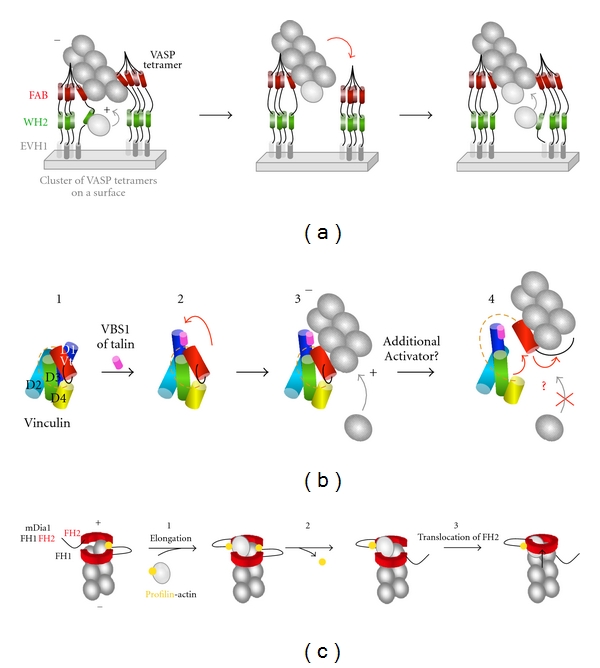
Regulation of actin assembly by VASP, vinculin, and formins. (a) VASP-mediated processive elongation of actin filaments. Clusters of VASP tetramers allow the WH2 domains of several VASP molecules to deliver actin monomers to actin filament barbed ends in a processive manner. This activity is accompanied by an acceleration of barbed end elongation. Between delivery events, VASP remains associated to the side of the filament via its FAB domain, adapted from [42, 43]. (+) and (−) indicate the barbed end and the pointed end of the actin filament. (b) Regulation of actin filament side binding and barbed-end capping by vinculin. In this scheme, subdomains of vinculin are represented as cylinders. The proline-rich region that links Vh and Vt is represented as an orange dotted line. (1) Vinculin is autoinhibited by an intramolecular interaction between Vh (D1 to D4) and Vt. The F-actin binding site located in Vt is masked by D1. (2) The binding of the VBS1 domain of talin disrupts the D1-Vt interaction (red arrow). Vt is unmasked and binds to the side of an actin filament. Barbed end elongation is still possible. (4) The disruption of additional unidentified contacts unmasks the C-terminal arm (black line) of Vt which caps the barbed end of the filament, adapted from [41]. (c) Formin-mediated processive elongation of actin filaments. In this scheme, only the FH1 (black line) and FH2 (red) domains are represented. (1) Addition of a profilin-actin subunit to the formin-bound barbed end. Each FH2 protomer of the formin dimer makes two contacts with the terminal actin subunits at a barbed end. (2) Profilin dissociates. (3) The translocation of one FH2 protomer releases one of the two actin-formin bonds to allow the addition of next profilin-actin subunit, adapted from [3].
