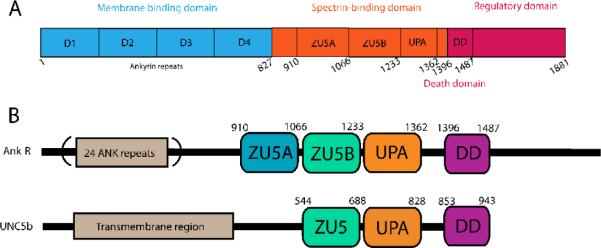
Figure 1.
A. Domain organization of canonical ankyrins. The ~89 kDa membrane binding domain consists of 24 tandem ankyrin repeats (blue), a 62 kDa spectrin-binding domain (orange) that includes the ZU5A, ZU5B, and UPA domains, and a 55 kDa C-terminal flexible regulatory domain (red) containing aDeath domain (DD). Numbering in the diagram corresponds to that of human ankyrin-R. B. Comparative domain organization of Ankyrin-R and UNC5b. UNC5b contains a large transmembrane region followed by ZU5 (light green), UPA (orange) and DD (purple) domains. The arrangement of these domains is similar to the one observed in ankyrins. In the latter, the first ZU5 domain (blue), ZU5A, mediates the interaction with β-spectrin repeats 14–15. UNC5b is known to form a supramodule involving ZU5-UPA-DD domains25. Based on the sequence similarity, it is expected that ankyrin forms a similar supramodule involving the ZU5B, UPA and DD domains, but not the ZU5A spectrin binding domain.