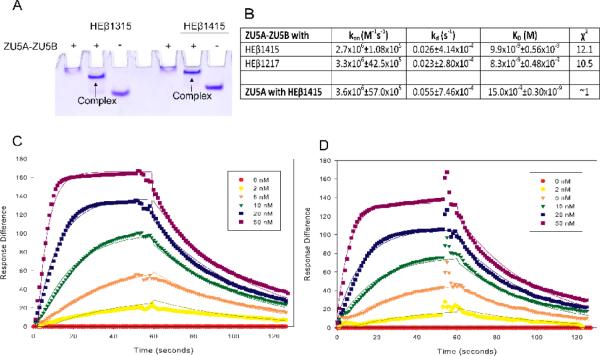
Figure 2. The ZU5B domain does not affect spectrin/ankyrin binding.
A. Native gel shift assay of the ZU5A-ZU5Bfragment of ankyrin and spectrin. The ZU5A-ZU5B fragment was mixed with repeats 13–15 (HEβ1315) or 14–15 (HEβ1415) of human erythrocyte β spectrin to ascertain whether the fragments interact. The native gel shift shows a shift in spectrin mobility due to the interaction with ankyrin. The presence of absence of ZU5A-ZU5B is shown by + or − signs. As expected, the ankyrin fragment interacts with spectrin repeats containing the ankyrin binding region. B. Binding affinity and kinetic parameters data describing the ZU5A-ZU5B interaction with β-spectrin.Binding affinity and kinetic parameters measured by SPR between the ZU5A-ZU5B fragment and repeats 12–17 (HEβ1217) or repeats 14–15 of human erythrocyte β-spectrin. For comparison, parameters for the ZU5A only fragment are shown in the final row of the table21. The ZU5A-ZU5B interaction with human β-spectrin is comparable to the one observed between spectrin and ZU5A alone. C and D. SPR trace of ZU5A-ZU5B interaction with HEβ1217 and HEβ1415. Surface plasmon resonance data and fits for ZU5A-ZU5B binding to biotinylated HEβ1217 or HEβ1415 at various concentrations of ZU5A-ZU5B shown in the inset.