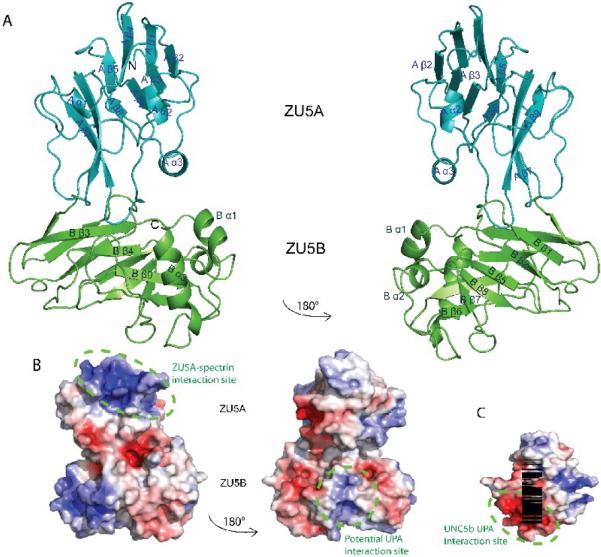
Figure 3. Structure of the ZU5A-ZU5B fragment of human erythrocyte ankyrin-R.
A. Ribbon diagram of the ZU5A-ZU5B fragment. N and C-termini are as labeled on figure. The structure shows two independent, compact, and similar well-folded β-sheet rich cores, each with different numbers of loops and helices. The ZU5B domain (green) extends away from the ZU5A (turquoise) and interacts minimally with it. The ZU5A and ZU5B domains do not interact extensively.The interface between the two domains is small and involves few contacts. The contact surface area is 514 Å231, emphasizing the minimal interaction between the domains. B. Electrostatic surface charge of ZU5A-ZU5B. The ZU5A-ZU5B fragment shows a positive patch on the surface on the ZU5A domain that corresponds to the spectrin binding site23; 24 (circled in purple). In contrast, ZU5B does not exhibit a marked charged character and does not have the equivalent positively charged patch associated with spectrin binding. C. Electrostatic surface charge of the ZU5 domain of UNC5b (PDB ID: 3G5B). The electrostatic map of the ZU5 domain of UNC5b displays a negative patch corresponding to the region of interaction with the UPA domain (circled in green). A similarly charged patch is observed on the surface of the ankyrin ZU5B domain, suggesting that the ankyrin UPA domain could make a similar interaction with ZU5B. The UNC5b domain is shown in the same orientation as the right panel of the ZU5A-ZU5B diagram for ease of comparison. The molecular surface of the molecule is shown with the equipotential electrostatic surface mapped onto it at ± 5 kBT/ec (blue and red, respectively)33.