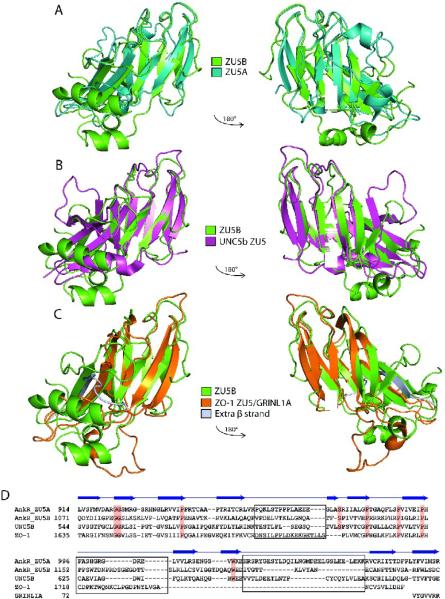
Figure 4. ZU5 domains have a similar structure containing a β-sheet core.
A. Superposed structures of the ankyrin ZU5A domain on the ZU5B domain. Ribbon diagram of ZU5A (turquoise) superposed on ZU5B (green). The C-terminus of the protein is labeled. The overall β-sheet rich core is similar in both structures, but ZU5B shows differences in the positioning of helices and loops. In addition, the ZU5B domain has an additional helix. B. Superposed structures of ankyrin ZU5B on the ZU5 domain of UNC5b (PDB ID: 3G5B). Ribbon diagram of ankyrin-R ZU5B (green) superposed on the ZU5 domain of UNC5b (pink). The N-terminus of ZU5B and C-terminus of ZU5 of UNC5b are labeled. The comparison shows a clear similarity in the β-sheet rich core, but ZU5B has a larger structure due to the presence of longer loops and two extra two helices. C. Superposed structures of ankyrin-R ZU5B on the ZU5 domain of ZO-1 in complex with GRINL1A peptide (PDB ID: 2KXS). Ribbon diagram of ZU5B of ankyrin-R (green) superposed on the ZU5 domain of ZO-1 in the ZO-1/GRINL1A complex (orange). The N-terminus of ZU5B and the N-terminus of ZU5/GRINL1A of ZO-1 are labeled. The ZO-1 ZU5 domain forms an incomplete ZU5 fold that is completed by a region at the N-terminus of the protein and the GRINL1A peptide at the C-terminus32. The comparison shows region that the ZO-1 ZU5 domain is structurally very similar to the ZU5 domain of ZU5A of ankyrin-R in the β core region. Extra residues (1–14) included in the ZO-1/GRINL1A structure, which are not part of the ZO-1 or GRINL1A sequence, are shown forming an extra β strand (silver). D.Structure-based sequence alignment of four known ZU5 domains: ZU5A and ZU5B of Ankyrin-R, ZU5 of UNC5b, and ZU5/GRINL1A of ZO-1. The sequence alignment is strictly based on structural similarities. Some regions of the proteins are structurally dissimilar (shown in black boxes) precluding sequence alignment. Highly-conserved residues across the ZU5 domains are shaded in red. The β-strands observed in the ZU5B domain of ankyrin-R are indicated above the sequences. The alignment of the GRINL1A peptide is also shown.