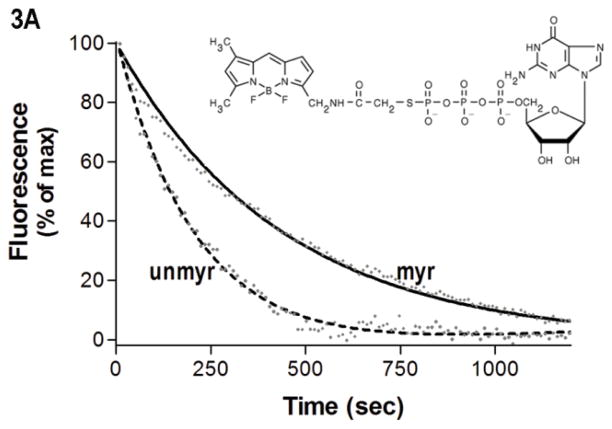
Fig. 3.
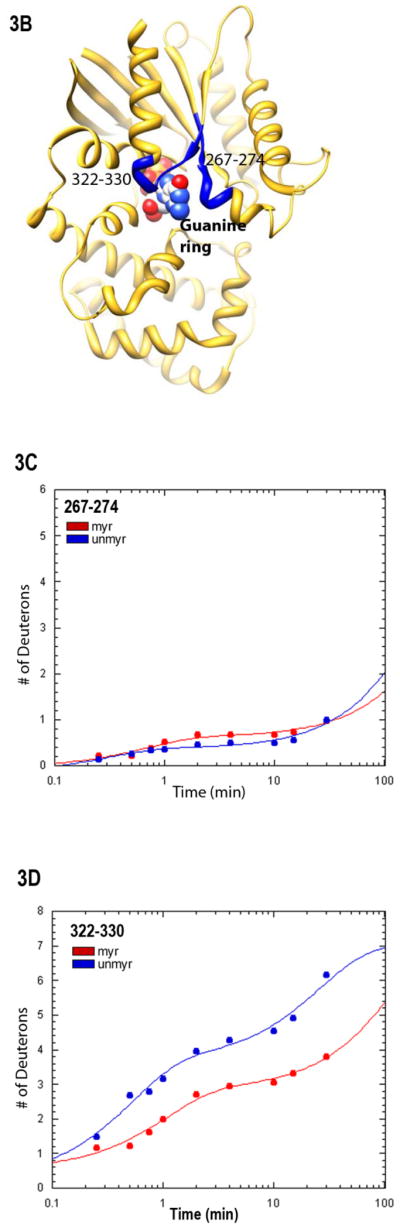
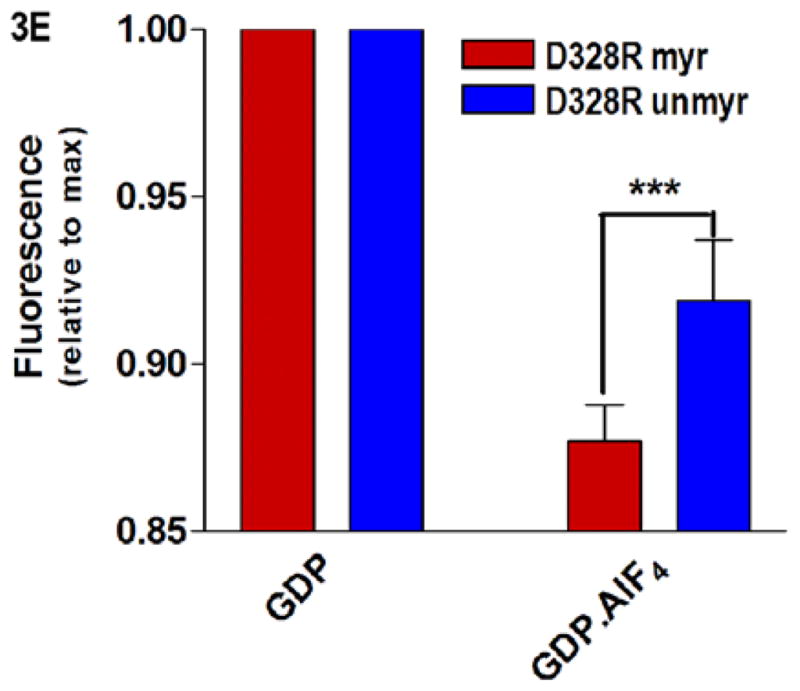
Protein-nucleotide binding interactions influenced by myristoylation. (A) GαiGDP proteins were preloaded with 1 μM BD-GTPγS for 1 hr, and dissociation initiated at T=0 by addition of 10 μM unlabeled GTPγS (emission monitored at ex/em 485/515 nm) as described in methods. Data points are the average of 3 independent experiments, which were fit to a single exponential dissociation curve using GraphPad Prism 4.0. Inset: structure of BD-GTPγS used in these studies (Invitrogen, Madison, WI). (B) Regions of Gαi that surround the guanine ring (1GFI (2), rendered with Chimera (66)) are shown as blue ribbons. (C–D) Time-dependence of H/D exchange (as described in Fig. 2) for peptides (C) 267–274 flanking the guanine ring and (D) 322–330 located at the base of the α5 helix. (E) Myr-dependent stabilization of interactions between guanine ring and residue 328 located at the base of the C-terminal α5 helix. Left two bars: maximal level of BD-GTPγS fluorescence (ex/em 485/515 nm) for each protein exhibited upon full BD-GTPγS binding measured after 1 hr (set to 1.0). Right two bars: pre-activation of GDP-bound proteins with 10 μM AlF4 for 5 minutes prior to addition of BD-GTPγS reduces exchange of GDP•AlF4 for BD-GTPγS, as compared to their respective maximums. Data are the average of 3 independent experiments; results are mean ± SEM.