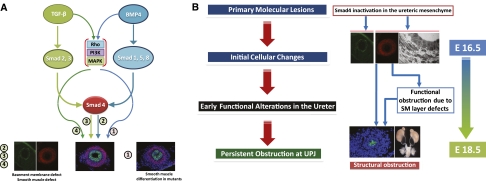
Figure 6.
Pathogenesis of UPJ obstruction in ureters of the Smad4 mutants. Because the SM layer development seems less affected in Smad4 mutants than Bmp4 mutants16 and TGF-β superfamily signaling may have Smad4-independent downstream pathways mediated by ρ-GTPase, phosphatidylinositol 3′ kinase, and MAPK, it is possible that the part of Bmp4 signaling in ureteral SM development may be Smad4-independent. (A) At the same time, other phenotypes in Smad4 mutants could be attributed to the lack of TGF-β/Bmp4 signaling by Smad4. Although the series of sequential and parallel events intertwine, the pathogenesis of UPJ obstruction in the Smad4 mutants seems to go through a few distinct phases. The primary molecular lesion is Smad4 inactivation in the ureteral mesenchyme. This inactivation causes initial cellular changes, including the reduction of SMC number and the change of contractility related proteins. These and other early changes lead to functional obstruction of the ureter. (B) Functional obstruction causes subsequent bending and luminal constriction of the ureter at UPJ, marking the transition from the earlier dysfunction to later, more intractable structural obstruction.