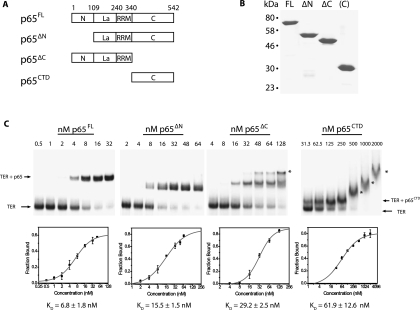
FIGURE 1.
(A) Schematic diagram demonstrating the domain organization of p65. p65 has four domains: an N-terminal domain, a La motif, an RNA recognition motif (RRM) domain, and a C-terminal domain. Protein constructs were expressed for full-length p65 (p65FL), p65 lacking the N-terminal domain (p65ΔN), p65 lacking the C-terminal domain (p65ΔC), and only the C-terminal domain of p65 (p65CTD). (B) A Coomassie stained SDS-PAGE gel of the four purified p65 constructs. Numbers on the left indicate the position of molecular weight markers. (C) Affinities of p65 constructs for telomerase RNA as determined by gel shift assay. Radiolabeled TER was incubated with increasing concentrations of p65FL, p65ΔN, p65ΔC, and p65CTD and run on a native polyacrylamide gel, top panels. Gels were quantified to determine the fraction bound (bottom panels). Error bars represent the standard deviation of triplicate measurements. The line is a fit of the data used to determine the dissociation constant (Kd) of each p65 construct for its TER substrate. The data were fit to the equation F = [(Fmax)(cn)]/[(Kd)n(cn)], where F represents the fraction bound, c represents the concentration, Kd is the dissociation constant (the concentration at which 50% of the RNA is bound), Fmax represents the maximal value of F, and n represents the Hill coefficient. The following Hill coefficients were obtained: p65FL n = 1.3, p65ΔN n = 1.3, p65ΔC n = 2.1, p65CTD n = 0.9. Asterisks mark higher-order protein-RNA complexes formed at high protein concentrations.