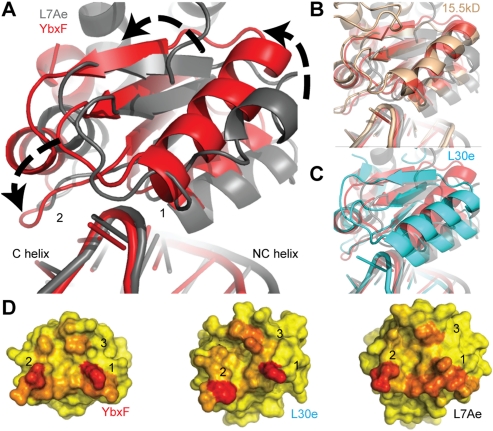
FIGURE 3.
Global comparison of K-turn-binding proteins bound to K-turns. (A) Alignment of K-turn G•A sheared pairs from RNP complexes (YbxF, red; L7Ae, gray) reveal ∼12° counterclockwise rotation of YbxF relative to L7Ae. (B) Eukaryal protein 15.5kD (brown) aligns closely with YbxF in its orientation on the K-turn, while (C) eukaryal protein L30e (cyan) has an intermediate degree of rotation. (D) Analysis of the three interaction surfaces reveals that L7Ae buries more surface area (red) than YbxF and L30e and is unique in its distribution of interacting residues. The accessible surface area for YbxF was calculated subsequent to the addition of side-chain rotamers similar to L7Ae at residues K17 and K21 (α2) and E72 (α4–β4 loop).