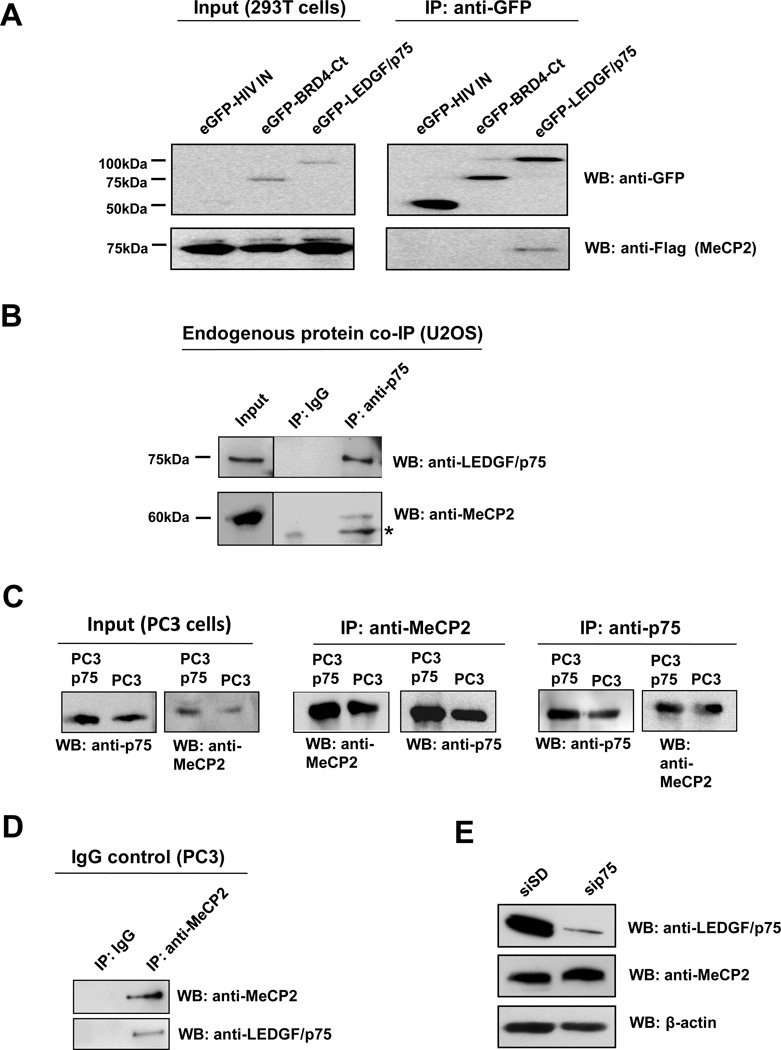
Figure 3. Co-immunoprecipitation of LEDGF/p75 and MeCP2.
(A) 293T cells co-transfected with Flag-MeCP2 and eGFP-LEDGF/p75, eGFP-HIV-IN, or eGFP-BRD4-Ct expression constructs were lysed 24 h post transfection. Proteins in cell lysates were immunoprecipitated (IP) with antibody against GFP, resolved by SDS-PAGE, and detected by immunoblotting using anti-GFP and anti-Flag antibodies. (B) Endogenous proteins in U2OS cell lysates were immunoprecipitated with mouse monoclonal anti-LEDGF/p75 antibody, as well as an irrelevant IgG as negative control. Immunoprecipitated proteins were detected by immunoblotting with rabbit anti-LEDGF/p75 and anti-MeCP2 antibodies. *Denotes degraded MeCP2. Protein input was determined by immunoblotting of whole cell extracts. (C) Proteins in lystates from PC3 cells or cells stably overexpressing LEDGF/p75 (PC3p75) were immunoprecipitated with rabbit anti-MeCP2 and mouse monoclonal anti-LEDGF/p75 antibodies. Immunoprecipitated proteins were detected by immunoblotting with rabbit anti-LEDGF/p75 and anti-MeCP2 antibodies. (D) Endogenous proteins in PC3 cell lysates were immunoprecipitated with rabbit anti-MeCP2 antibody, as well as an irrelevant IgG as negative control. Immunoprecipitated proteins were detected by immunoblotting with anti-LEDGF/p75 and anti-MeCP2 antibodies. (E) Immunoblot of total proteins from PC3 cells with (sip75) and without (siSD) siRNA-mediated knockdown, using rabbit antibodies to LEDGF/p75 and MeCP2.