Figure 6. Oxidized mitochondrial DNA binds to NLRP3 and activates the inflammasome.
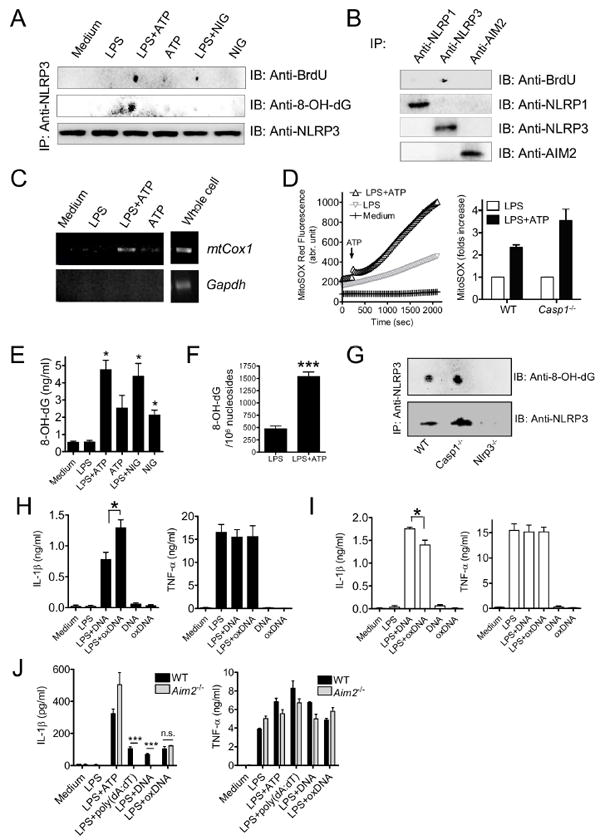
(A) BMDM were preloaded with BrdU (10 μM), and cells were treated with LPS (1 μg/ml, 3 hr), followed by 3-MA (2.5 mM, 1 hr), ATP (5 mM, 1 h) or NIG (10 μM, 1 h). Cell lysates were collected and immunoprecipitated with anti-NLRP3 Ab, then detected by dot-blots probed with anti-BrdU or anti-8OH-dG Abs. As a loading control, NLRP3 immunoblot was performed.
(B) BrdU-preloaded BMDM were treated with LPS+ATP, cell lysates were immunoprecipitated with Ab against NLRP1, NRLP3, or AIM2. Immune dot-blots with BrdU Ab, and respective control immunoblots are shown.
(C) Mitochondrial COX1 DNA associates with NLRP3 immunoprecipitates. COX1 DNA was amplified from the NLRP3 immunoprecipitates by PCR.
(D) BMDM were stimulated with or without LPS for 3 hr, then loaded with 2.5 uM MitoSOX for 20 min. Mitochondrial ROS was measured every 30 s thereafter. ATP was added 240 s after monitoring was initiated. Relative mitochondrial ROS increase at 30 min after ATP was shown.
(E and F) Mitochondrial DNA was extracted from BMDM after NLRP3 inflammasome stimulation, and amounts of 8-OH-dG were quantified by (E) ELISA and (F) LC-MS-MS (means ± SD).
(G) 8-OH-dG immune dot-blots were performed in WT, Casp1–/– and Nlrp3–/– BMDM after LPS+ATP treatment. LPS-primed BMDM were treated with 3MA for 2 hr, followed by stimulation with ATP for 30 min. Cell lysates were collected and NLRP3 Immunoprecipitation and 8-OH-dG dot-blot were performed in WT, Casp1–/– and Nlrp3–/– BMDM.
(H and I) LPS-primed (H) WT or (I) Nlrp3–/– BMDM were treated with exogenous 8-OH-dG incorporated DNA (oxDNA, 2 μg/ml) or control DNA for 8 hr. IL-1β and TNFα amounts were quantified in supernatants by ELISA (means ± SD).
(J) LPS-primed Aim2–/– BMDM were treated with exogenous oxDNA (2 μg/ml) and IL-1β and TNFα concentrations were determined in culture supernatants by ELISA (means ± SD).
