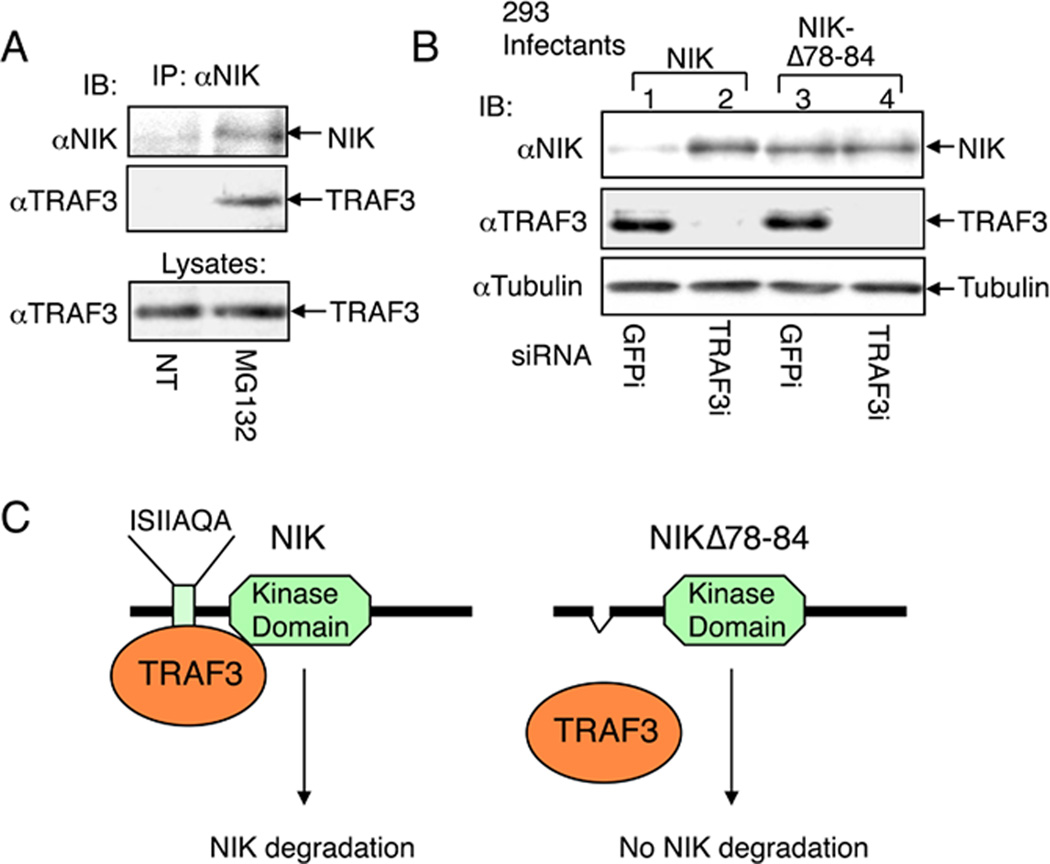
Fig. 2. NIK degradation through NIK-TRAF3 association.
(A) M12 B cells were either not treated (NT) or incubated for 2 h with proteasome inhibitor MG132. The NIK protein complex was isolated by IP followed by detection of NIK (top panel) and TRAF3 (middle panel) by IB using anti-NIK and horseradish peroxidase-conjugated anti-TRAF3, respectively. The TRAF3 expression level was detected by direct IB (bottom level). (B) 293 cells were infected with retroviruses encoding NIK or a NIK mutant lacking the core region of TRAF3-binding site (NIKΔ78–84). Stably infected cells were transfected with small-interfering RNA (siRNA) for either the control GFP or TRAF3. Cell lysates were subjected to IB using the indicated antibodies to detect the expression level of NIK (top panel), TRAF3 (panel 2), or control tubulin (panel 3). (C) Schematic picture depicting the regulation of NIK degradation by TRAF3. Wildtype NIK is bound by TRAF3 via an N-terminal domain and targeted for continuous degradation, which explains why NIK is barely detectable unless when the cells are treated with a proteasome inhibitor (A) or TRAF3 siRNA (B). NIKΔ78–84 is not bound by TRAF3 and stable even in the presence of TRAF3. This figure is adapted from Liao et al. (29) with permission.