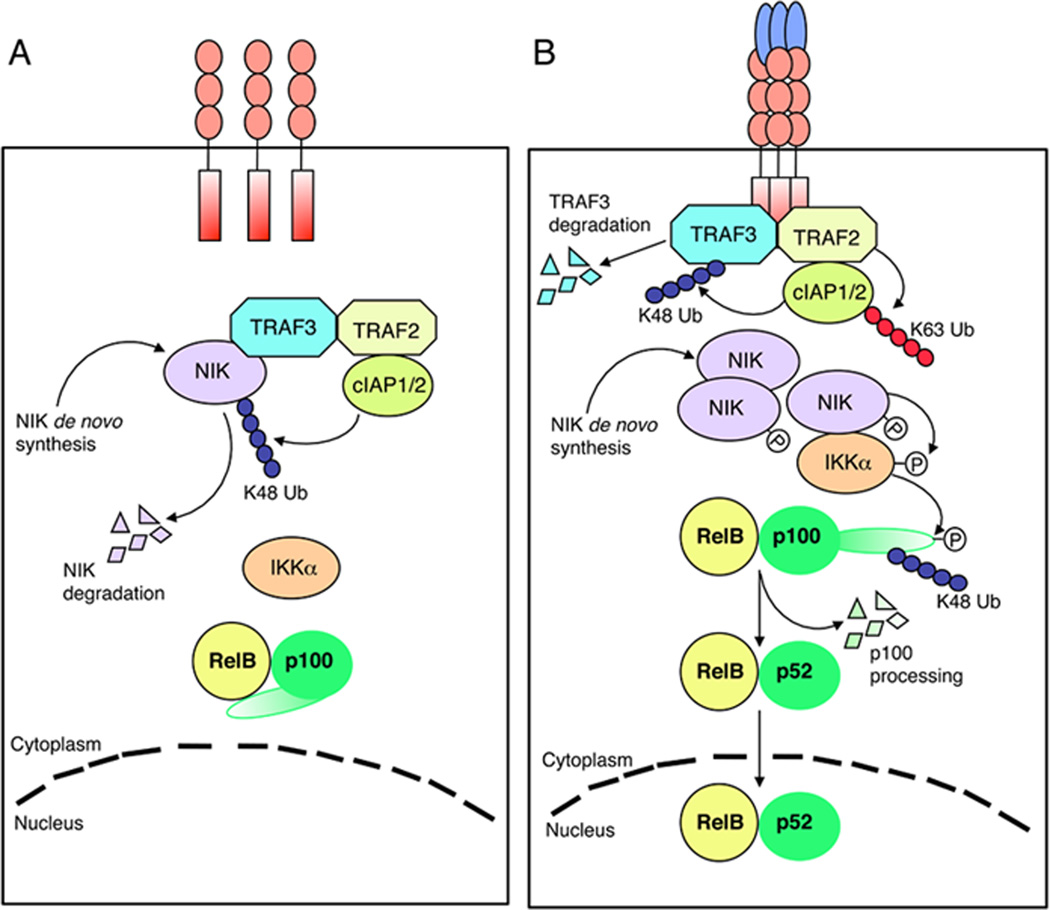
Fig. 4. A model of noncanonical NF-κB regulation.
(A) Under normal conditions, de novo synthesized NIK is immediately bound by TRAF3 and recruited to the TRAF-cIAP E3 ubiquitin ligase complex via TRAF3-TRAF2 dimerization. cIAP1/2 catalyzes K48 ubiquitination of NIK, targeting NIK for degradation in the proteasome. The continuous degradation of NIK prevents NIK accumulation or activation of noncanonical NF-κB signaling. (B) Ligand engagement of the noncanonical NF-κB-stimulatory receptors induces recruitment of the TRAF-cIAP E3 components to the receptor complex. Probably due to its aggregation, TRAF2 becomes activated and mediates K63 ubiquitination of cIAP1/2, a modification that stimulates the K48-specific E3 activity of cIAP1/2. cIAP1/2 then conjugates K48 polyubiquitin chains to TRAF3, resulting in TRAF3 degradation. In the absence of TRAF3, the de novo synthesized NIK is stabilized and accumulated to higher levels; this allows NIK to induce IKKα-dependent p100 phosphorylation and ubiquitination, leading to the nuclear translocation of the generated RelB/p52 heterodimer.