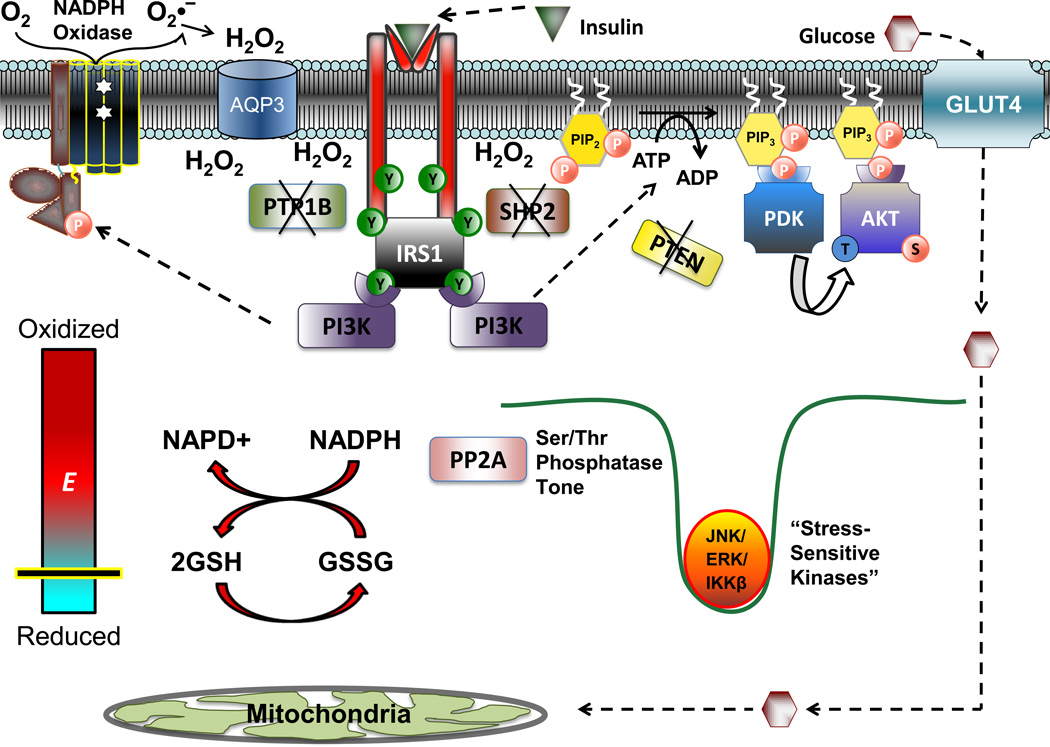
Figure 5. Proposed alterations in cell redox as a necessary component of insulin signaling.
In the absence of insulin, the principle nodes of regulation within the insulin signaling cascade are kept dephosphorylated via the membrane associated proteins PTP1B, SHP2, and PTEN. Following insulin binding and Tyr-phosphorylation of the insulin receptor and IRS1, the activation of membrane bound NADPH oxidase (potentially mediated by PI3K) results in the accumulation of H2O2 at the level of the plasma membrane to transiently inactivate PTP1B, SHP2 and PTEN, thus allowing propagation of kinase-mediated signaling, leading to GLUT4 translocation and glucose uptake. While the local redox environment at the plasma membrane, and redox sensitive proteins in direct proximity to the H2O2 source undergo an oxidative shift, the global redox state of the cell is maintained by the redox buffering systems(depicted by the thick red arrows illustrating the reaction between the 2GSH/GSSG and NADPH/NADP+couples). Maintenance of global redox ensures that Ser/Thr phosphatase activity, specifically PP2A (and potentially MKP and PP1, not shown), are maintained. The continued activation of these enzymes ensures that certain Ser/Thr kinases (JNK, ERK, IKKβ) remain inactive and that phosphomoieties are not allowed to accumulate within insulin signaling proteins.