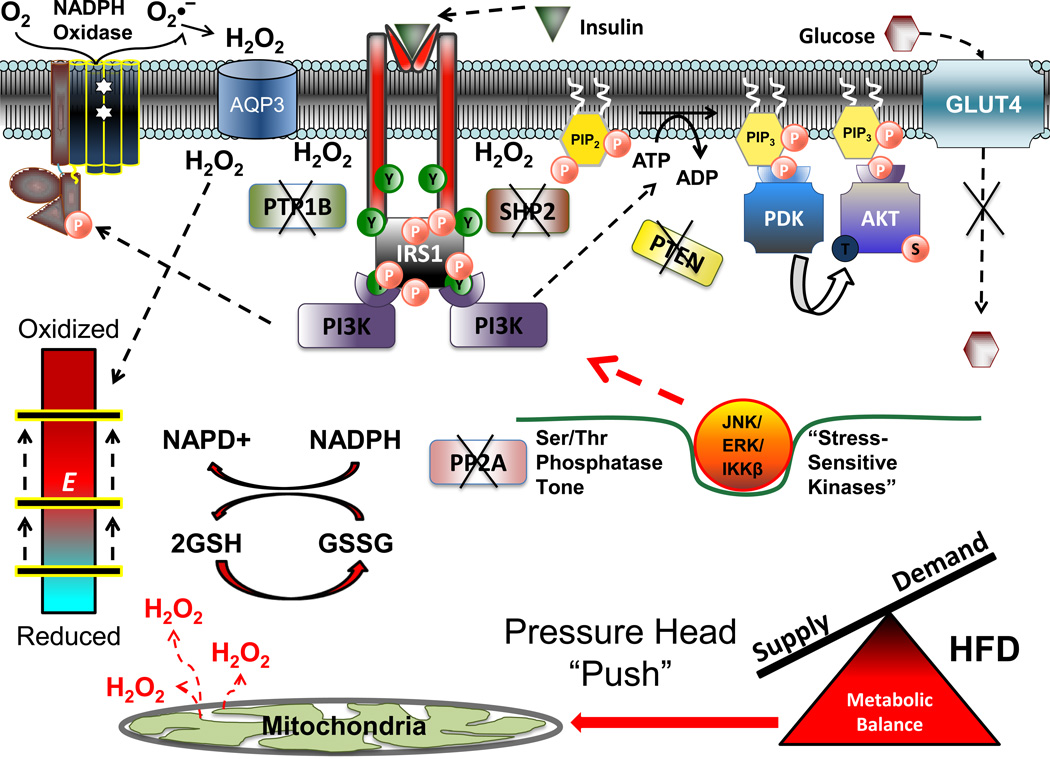
Figure 6. Proposed model of high fat diet-induced insulin resistance.
Chronic elevations in mitochondrial H2O2 emission as a result of a high fat diet serve to reduce the reserve capacity within the redox buffering systems and induce an oxidative shift in the cellular redox environment (bar shifted up to beginning of red zone on redox environment gauge). This global shift in cell redox may be sufficient to inactivate cellular Ser/Thr phosphatase (PP2A) activity, in turn promoting activation of stress-sensitive Ser/Thr kinases (JNK, ERK, IKKβ) and accumulation of inhibitory phosphomoieties within various insulin signaling proteins. Alternatively, the oxidative shift in global redox environment induced by the high fat diet may compromise the capacity of the redox buffering systems to buffer the H2O2 production from membrane bound NADPH oxidase in response to insulin, resulting in a further shift in global redox environment that is sufficient to inactivate both PTP and Ser/Thr phosphatases, all of which decreases signal propagation throughout the cascade and impairs glucose uptake.