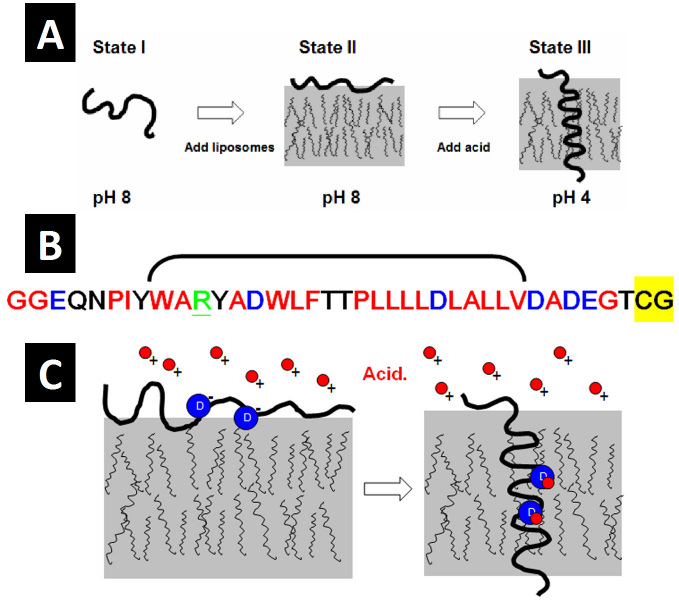
Figure 1.
A) pHLIP exists in three biophysical states depending on context: State I, as a largely unstructured monomer in aqueous solution at slightly basic or neutral pH; State II, as a largely unstructured monomer partitioned to the membrane surface in the presence of liposomes; and State III, as an inserted, bilayer-spanning α-helix at low pH. All states are reversible as denoted by the double arrows. Adapted from Biophys J. 2007;93(7):2363-72 [17]. B) The primary sequence of pHLIP. Red indicates hydrophobic residues, black indicates polar residues, blue indicates acidic residues, and underlined green indicates basic residues. The bracket denotes residues at positions 9-30 containing the two aspartic acids (blue D) at positions 14 and 25. The highlighted yellow residues comprise the cysteine tag attached to the inserting end of the peptide for use in conjugating cargo molecules for translocation into cells. C) The mechanism of pHLIP insertion at low pH: Insertion is largely dependent on the titration of the two aspartic acids (blue circles) at positions 14 and 25. Acidic protons (red circles) bind to the basic aspartic side chains at low pH, neutralizing the charge and converting the side chains into a more hydrophobic form. This thermodynamic conversion facilitates insertion by lowering the energy barrier (by about 3 kCal/mol [13]) for stable interaction with the membrane bilayer.