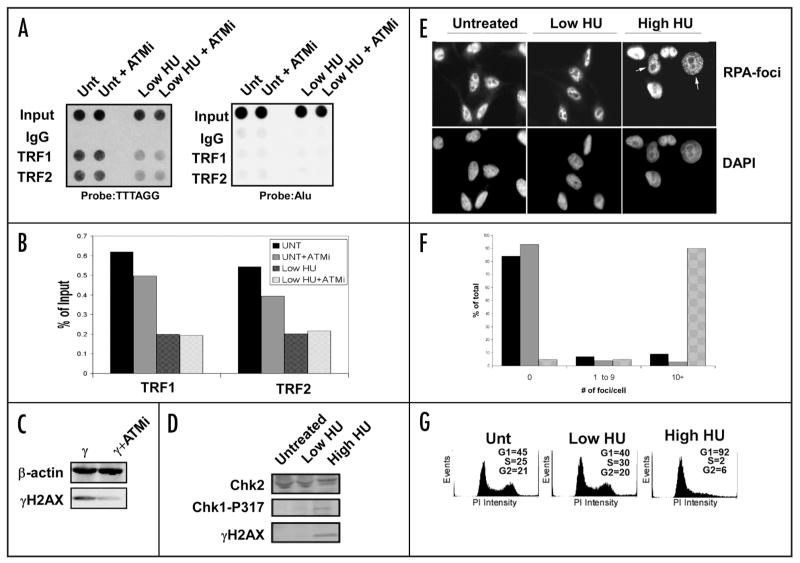
Figure 6.
Lack of DNA damage response after low dose HU treatment. (A) Raji cells were treated with the ATM inhibitor KU-55933 (10 μM, 24 h) with or without HU and used in ChIP experiments to determine the efficiency of TRF1 and TRF2 binding to telomeres. An Alu probe was used as a control. (B) Quantification of the data collected in (A). (C) Western blot of the common DNA damage marker and ATM target γH2AX after treatment with the ATM inhibitor. The cells were irradiated with 5 Gy γIR and harvested 15 min after treatment. β-actin is shown as a loading control. (D) Western blot of Raji cell extracts treated with low HU show little induction of common replication stress or damage markers. A high dose of HU (1 mM) is used as a positive control. A Chk2 western blot is used as a loading control. (E) Low HU treatment does not induce RPA foci after treatment. HeLa cells were treated with low HU for 1 d and then used for immunofluoresence to determine if RPA is recruited to sites of replication stress after treatment. A high dose (1 mM) sample is included as a control. All nuclei were stained with DAPI as a counterstain. White arrows indicate cells containing >10 RPA-foci. (F) Quantification of the data generated in (E). One hundred nuclei were counted for each condition shown. Untreated cells (black bars), low HU (gray bars), or high HU (patterned bars) were scored for 0, 1–9 or >10 foci per cell, as indicated. (G) Propidium iodide staining of HU-treated cells shows no drastic shift in cell cycle profile after treatment. A high dose HU sample (1 mM) is included as a positive control. Quantification of cells in each phase of the cell cycle is presented for each panel.