Figure 6.
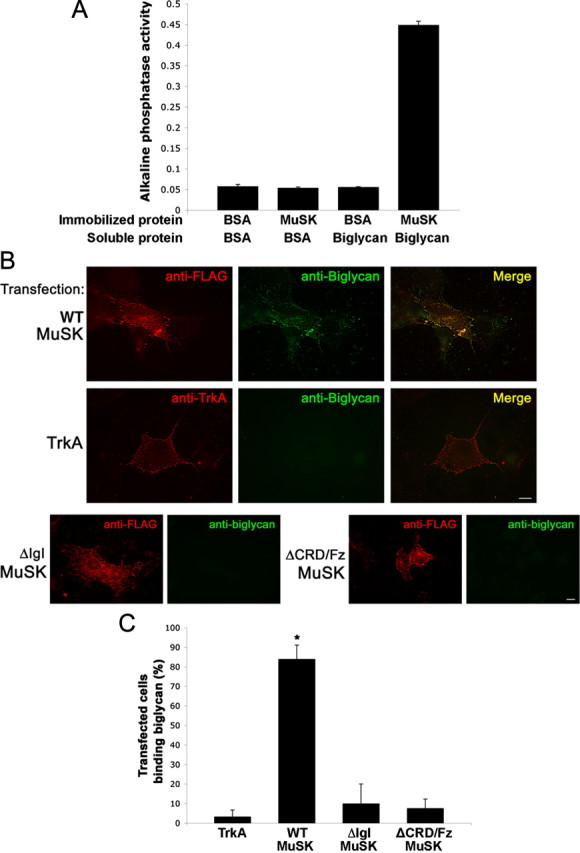
Biglycan binds to MuSK. A, Direct binding of biglycan and MuSK. Purified MuSK ectodomain or BSA was immobilized on plastic wells and then incubated with either BSA or with 500 ng of biotinylated recombinant biglycan followed by avidin-alkaline phosphatase. Biglycan binds to MuSK but not to BSA (0.45 ± 0.01 and 0.06 ± 0.01, respectively; n = 3, Student's unpaired t test, p < 0.01). B, Biglycan binds to MuSK expressed in heterologous cells. COS cells were transfected for 24 h with FLAG-tagged full-length wild-type MuSK (WT-MuSK), TrkA, or FLAG-tagged MuSK mutants lacking either the IgI (ΔIgI) or CRD/Fz domain (ΔCRD/Fz) as indicated. Live cells were incubated with purified recombinant biglycan polypeptide (20 nm) for 30 min at 4°C and bound biglycan was visualized with an anti-biglycan monoclonal antibody and Alexa488-conjugated secondary antibodies (green) and then fixed. The transfected constructs (red) were visualized with anti-FLAG (all MuSK constructs) or anti-trkA ectodomain. Note that biglycan binds to COS cells expressing full-length, wild-type MuSK (top), but not to cells expressing TrkA (middle), mutant MuSK ΔIgI, or ΔCRD/Fz (bottom). No binding was detected to untransfected cells (data not shown). Scale bars, 10 μm. C, Quantification of biglycan binding to MuSK or TrkA transfected cells. Data are expressed as the mean percentage of cells that bound biglycan per microscope field Little biglycan binding was detected on cells expressing heterologous TrkA (3.3 ± 3.3%; n = 10 fields). Cells expressing wild-type MuSK exhibit robust biglycan binding (86.3 ± 7.1%; n = 10 fields; *p < 0.001, Students unpaired t test). Biglycan binding to cells expressing MuSK-ΔIgI or ΔCRD/Fz was not significantly different from that to TrKA-expressing cells; p > 0.8, one-way ANOVA).
