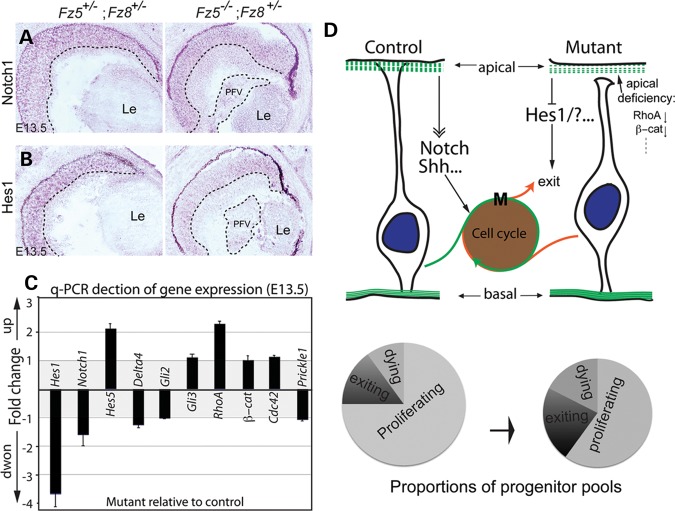
Figure 7.
Altered gene expression of signaling pathways. (A and B) In situ hybridization at E13.5 shows that although Notch1 expression is essentially unaffected (A), its downstream effector, Hes1, is grossly reduced through the mutant retina (B). The dashed lines demarcated the boundary between INBL, ONBL and PFV tissue. (C) q-PCR detection of gene expression involving Notch and Shh signaling and apical-basal polarity. Consistent with in situ hybridization, Hes1 expression is downregulated in the mutant retina ∼3.5-fold (P< 0.01). There is a moderate upregulation of RhoA expression (P= 0.02) and unchanged β-catenin expression in the mutant retina. The expression of Shh downstream effectors, Gli2 and Gli3, was not altered. Y-axis: fold change of gene expression in the mutant retina with respect to control. Positive values indicate upregulation, while negative values indicate downregulation. Value of either ‘−1’ or ‘1’ indicates no changes. There are no values between −1 and 1 since the expression ratios <1 were treated with negative reciprocal, and plotted along the downregulation direction. (D) A schematic model for Fz5 and Fz8 functions during retinal neurogenesis. During the retinal neurogenesis, Fz8 and Fz5 are critical for neuroblast apical junction maintenance through a set of apical complex proteins, which include RhoA and β-catenin. This is important for the neuroblast polarity and organization. In the Fz5−/−;Fz8+/− mutant retina, the retinal apical deficiency subsequently affect Notch and/or Shh signaling components, of which Hes-1 is an example. This in turn pushes more progenitors to exit cell cycle. In such, the size of the proliferating progenitor pool in the mutant retina becomes smaller (see schematic pie charts), which is likely the cause of microphthalmia and retinal coloboma.